It seems that avid reef aquaria hobbyists are constantly in search of a better lighting system. Perhaps one is motivated by a desire for more rapid coral growth, or simply an aquarium that is more pleasing to the eye. The question “What is the best lamp?” is often asked. Although the question is valid, the “best lamp” means different things among hobbyists. Is an aesthetically pleasing aquarium the goal, or is the promotion of photosynthesis the ultimate objective? The former is purely subjective. Finding an answer to the latter is quantifiable, but requires some rather sophisticated equipment. This article suggests an answer to the photosynthesis issue based on results of experiments conducted with a newly available research instrument.
Those factors promoting photosynthesis must be given serious attention since most tropical corals of interest to hobbyists contain symbiotic zooxanthellae algal cells. Of these factors, lighting is of primary importance. Debates have raged over which parameter, light intensity or spectral quality is more important. Both, of course, play a part in promoting photosynthesis in zooxanthellae. Light intensity or Photosynthetic Photon Flux Density (or PPFD, simply the number of light particles – photons – falling upon a given surface) must meet zooxanthellaes’ minimal requirements or the algal cells eventually die. If spectral quality is not correct, photosynthesis is not promoted
and zooxanthellae become “starved” for proper light and will soon perish.
Estimating the spectral requirements of zooxanthellae is not particularly easy. Zooxanthellae contain various photosynthetic pigments, including chlorophyll A, chlorophyll C2, the carotenoid peridinin and perhaps others (all of which may be in varying proportions due to the photoadaptive capabilities of zooxanthellae). However, researchers have established the quality of light absorbed by these pigments and we can safely assume certain wavelengths are required.
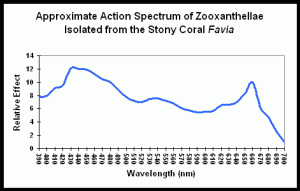
Figure 1 demonstrates the light energy harvested by zooxanthellae isolated from the stony coral Favia, in a chart called an “action spectrum.” An action spectrum describes the relative effectiveness of energy at different wavelengths in producing particular biochemical or biological responses (such as oxygen evolution, carbon uptake, electron transport rate, etc., during photosynthesis).
To believe that blue (430-480 nm) and red (600- 700 nm) wavelengths are required is only partially true. As Figure 1 demonstrates, a wide range of wavelengths are absorbed by chlorophylls A and C2; however, peridinin and perhaps other photopigments, effectively harvest light energy outside of the range normally associated with photosynthesis.
Researchers have addressed light quality and its effects on zooxanthellae and coral growth. Perhaps the most interesting is a paper by Kinzie et al. (1984); they presented evidence that corals grown more rapidly under blue and white light of the same intensities (~12% of solar Photosynthetically Active Radiation – PAR, ~250 µMols·m2·sec, or 10,000 lux) than under “green” or “red” light of equal intensities. These scientists used clear or colored acrylic filters and natural sunlight. The blue filter transmitted wavelengths of ~ 400 to 500 nm and the clear filter (transmission quality not shown in the paper) likely was a fair representation of sunlight (although most acrylics attenuate all wavelengths but tend to decrease violet and blue disproportionately). “Blue” light is suggested to have some rather “magical” properties – it has been noted to increase rates of protein synthesis in some algae, as well as cause shifts in photosynthetic pigment concentrations in zooxanthellae. Blue light has also been reported to increase rates of photosynthesis (Kinzie and Hunter, 1987). Are spectral characteristics of “blue” metal halide lamps sufficient to promote photosynthesis more efficiently in zooxanthellae of captive corals?
Unfortunately, the spectral qualities of light transmitted by these researchers’ filters only faintly resemble those of lights used over aquaria. It is a leap of faith to apply the results obtained under filtered sunlight to artificial light sources, which have spectral spikes. However, this has not stopped many from interpreting that higher Kelvin lamps are best for promoting photosynthesis in corals.
In an excellent series of articles, Joshi and Morgan (1998; 1999) presented spectral qualities of many metal halide lamps commonly sold in the pet industry, but stopped short of making recommendations to hobbyists. So, the question remains – are there major advantages to zooxanthellae/corals when using certain lamps, or is there only aesthetic appeal? Do common lamps with output weighted in the violet/blue regions of the spectrum and readily available to hobbyists actually increase the rates of photosynthesis?
Two lamps were chosen for use in an experiment designed to determine if differing spectralqualities do indeed make a difference in photosynthesis rates. The first lamp is a Philips 175-watt 4,000° K metal halide lamp (usually available for less than $20 in major home improvement centers). The second lamp is an Aquarium Lighting Systems 175-watt 12,000°K “Sunburst” metal halide lamp. Spectral signatures of these lamps were determined with an Ocean Optics spectrometer. Spectral compositions were estimated by use of a LiCor quantum meter and glass cut-off filters. Use of these filters provides reasonable estimates of violet and blue wavelengths (400-465 nm) and red wavelengths (600-700 nm). These filters transmit few wavelengths in the yellow and orange portion of the spectrum. Considering that metal halide lamps have spectral spikes at 575 and 577 nm (due to the element mercury contained within the arc tubes), the percentages of blue radiation shown in the pie charts are slightly overstated (See Figures 2 – 5). However, the Sunburst 12,000°K lamp is the “bluest;” the Philips lamp less so.
There are many ways to estimate the effectiveness of light on corals. If one has the time and patience, simple observations of growth (along with rigorous control of other factors) may
suffice. A more sophisticated approach is one using a respirometer and delta oxygen evolution as the metric in judging rates of photosynthesis. Preliminary results suggested there is
no benefit to photosynthesis when using a 20,000°K metal halide lamp as opposed to the use of an inexpensive halide lamp (Riddle and Amussen, 1999). However, respirometry is an inexact science, fraught with all the drawbacks of experiments conducted in small, sealed experimental chambers.
A new technique is now available – that of Pulsed Amplitude Modulation (PAM) fluorometry. This experiment employed a Mini-PAM meter, manufactured by Walz GmbH, Germany. This method is non-intrusive and is gaining acceptance as the preferred method of measuring rates of photosynthesis (Beer et al., 1998). The Mini-PAM measures the fluorescence yield of the chlorophyll A molecules in the photosystem of zooxanthellae in response to changes in illumination. Chlorophyll fluorescence is assumed to arise from reradiation of absorbed light energy from Photosystem II (PS II) antenna pigments (including chlorophyll A, chlorophyll C2, and peridinin).
Fluorescence and the photochemical reactions of photosynthesis are competing processes in the dissipation of absorbed light energy. Energy absorbed by antenna pigments is generally assumed to have three primary pathways for dissipation (see Figure 6). First, it can be reradiated (fluoresced); second, it can be dissipated as heat or, third, it can be transferred to the reaction center of PS II. Once in the reaction center, this energy is available for use in photochemistry. Reduction-oxidation potential of the primary quinone acceptor (QA) governs what happens next. If the Qa is oxidized (the reaction center is said to be “open”), a photochemical reaction will occur and eventually lead to oxygen evolution and carbon fixation, the events that we associate with photosynthesis. However, if the QA is reduced (the reaction center is “closed”) the energy cannot be used in photochemistry. Therefore the chances of thermal dissipation and fluorescence will increase.
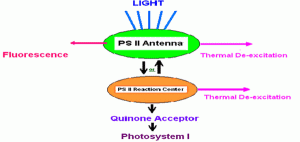
Figure 6. Assumed potential pathways for energy in Photosystem II. See text. After Olaizola and Yamamoto, 1994.
Thus, the magnitude of the fluorescence signal depends mainly on the amount of light energy absorbed (which itself depends on the spectral quality and intensity of the illumination source and the quantity and absorption spectra of the photosynthetic pigments present in the cells) and the fraction of reaction centers that are open.
The Mini-PAM exploits the relationship between photochemistry and fluorescence, and how it changes under different illumination conditions, to estimate the capacity of photosynthetic cells to photosynthesize (i.e., the fraction of reaction centers that are open). Essentially the Mini-PAM estimates the fraction of reaction centers that are open by comparing the magnitude of the fluorescence signal under ambient illumination (e.g., different lamps or sunlight) and the magnitude of the fluorescence signal following a saturating flash of light that temporarily overwhelms PS II and closes all the reaction centers.
Over time scales of several seconds/minutes we can assume that the pigment content of the cells does not change. Thus, if one were to measure the changes in fluorescence emitted by chlorophyll at a given spot on a coral induced by different amounts of light, an estimation of photochemical efficiency can be estimated. If one were to measure the fluorescence of this same spot when exposed to different spectral qualities (but at given light intensities), an estimation can be made of light sources’ ability to promote photosynthesis and hence comparisons can be made. In essence, this meter indirectly measures changes in the electron transport rate of PS II under different illumination conditions.
In order to determine if light quality makes a difference in the rate of photosynthesis, the quantity of light must be equivalent in each portion of the trials. A jig, holding the lamps, allowed quick and easy adjustments of light intensity to predetermined levels. The submersible probe of an Apogee Instruments quantum meter was placed immediately next to the coral used in the experiments and measured light energy, more specifically Photosynthetically Active Radiation (PAR).
The coral chosen for the experiment is one popular with hobbyists – the “Mushroom” coral (Fungia scutaria). This coral is usually maintained in a 300-gallon system with flow-through of natural seawater. Natural sunlight is the light source and is attenuated with shade cloth. Maximum light intensity, as measured at noon and at the coral’s surface is about 17% of natural sunlight’s visible energy – ~350 µMols·m2·sec, or ~17,500 lux. The relatively flat shape of the Fungia specimen allowed use of a shallow water basin and easy positioning of the PAM probe.
Results and Discussion
The Mini-PAM meter has the ability to collect and store much information, including time and date, minimum and maximum chlorophyll fluorescence, photosynthetically active radiation, yield, etc. Data obtained during the experiment was downloaded and analyzed.
Photosynthesis boils down to a flow of electrons, therefore the Electron Transport Rate (ETR) is indicative of the rate of photosynthesis. Calculation of the ETR is straightforward and is as follows: Quantum Yield (Y) multiplied by photosynthetic photon flux density (PPFD) absorbed by the Photosystem = ETR (Ilan and Beer, 1999). We did not conduct measurements of absorbed PPFD but we made the assumption that the pigment content of the coral’s zooxanthellae did not change during the experiment. Thus we report the “relative” ETR (not the absolute ETR). We also made the assumption that the ETR is zero during total darkness.
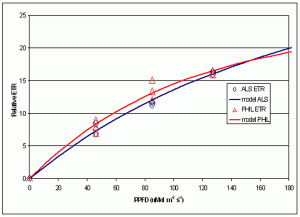
Figure 7. Comparison of Relative Electron Transport Rate in zooxanthellae under two different lamps at different PPFDs.
A portion of the information gathered is summarized in Figure 7, which shows the relative ETR measured under three difference PPFD intensities for each lamp (46, 85 and 127 mMols m-2 s-1- light intensities that are quite common at the bottom of aquaria where Fungia specimens are often placed).
This experiment’s results suggest information potentially valuable for hobbyists – that rates of photosynthesis were essentially the same under these two distinctly different light sources. Other than aesthetic value, there appears to be no advantage, photosynthetically speaking, in using high Kelvin lamps.
The implication of these results should be of interest to hobbyists; it suggests that lamp selection (with due regard to lamp intensity) may be based on appeal, whether that is price or
the “look” it gives to a tank, without fear of hindering photosynthesis. Economy-minded hobbyists and coral farmers may find this especially useful. It appears that light intensity and relatively simple light measurements alone adequately judge lamp efficiencies within the context of zooxanthellae photosynthesis. This should not be construed to mean that all light sources are adequate for reef aquaria use.
The spectral signatures obtained with the spectrometer demonstrate that these two metal halide lamps are full spectrum (though the 12,000° K lamp output is skewed towards the blue portion of the spectrum) and most resembles the “white light” category defined by Kinzie et al. (1984). Results garnered with the PAM meter suggest these two lamps are more or less equally efficient in the promotion of photosynthesis when PPFD values are the same.
It is inappropriate to claim that there are no major differences among the plethora of lamps available and their abilities to promote photosynthesis. Certainly the depreciation of overall lamp light output (PPFD) should be considered and readers are encouraged to review the works of Joshi and Morgan (1998; 1999, 2000) and others. Future experiments involving spectral quality and its effects should include more data points, different lamps and perhaps different coral species. Clearly, more work is required before we have an answer to the “best
lamp” question. For now, it appears that spectral quality might be subordinate to lamp intensity.
Special thanks go to Alexander Diffley for assistance in collection of the data.
References
- Beer, S., M. Ilan, A. Eshel, A. Weil and I. Brickner, 1998. Use of pulse amplitude modulated (PAM) fluorometry for in situ measurements of photosynthesis in two Red Sea faviid corals. Mar. Biol., 131(4): 607-612.
- Ilan, M. and S. Beer, 1999. A new technique for non-intrusive in situ measurements of symbiotic photosynthesis. Coral Reefs 18:
1:74. - Joshi, S. and D. Morgan, 1998. Spectral analysis of metal halide lamps used in the reef aquarium hobby, Part I. Aquarium Frontiers Online. November.
- Joshi, S. and D. Morgan, 1999. Spectral analysis of metal halide lamps used in the reef aquarium hobby, Part II. Aquarium Frontiers Online. January.
- Joshi, S., 2001. An analysis of recent metal halide lamps: Shedding some light on new reef tank illumination. Marine Fish and Reef USA, 2002 Annual. Fancy Publications, Irvine, Ca. pp. 56-69.
- Kinzie, R.A. and T. Hunter, 1987. Effect of light quality on photosynthesis of the reef coral Montipora verrucosa. Mar. Biol., 94:95-109.
- Kinzie, R.A., P.L. Jokiel and R. York, 1984. Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Mar. Biol., 78:239-248.
- Muscatine, L., 1980. Productivity of zooxanthellae. In: Falkowski, P.G. (ed). Primary Productivity in the Sea. Plenum Press, New York. Pp. 381-402.
- Olaizola, M. and H. Yamamoto, 1994. Short-term response of the diadinoxanthin cycle and fluorescence yield to high radiation in Chaetoceros muelleri (Bacillariophyceae). J. Phycol. 30: 606-612.
- Riddle, D. and A. Amussen, 1999. Spectrum or intensity? Proc. 1st Int. Conf. on Marine Ornamentals, Kailua-Kona, Hawaii. Page 70




0 Comments