Department of Biology and Biochemistry, Division in Ecology and Evolution, University of Houston
One of the greatest frustrations for aquarists is determining the proper conditions for their corals to thrive. There are many variables, not the least of which are proper light and water flow. Recommendations abound in the popular literature, and these are not always accurate. With some effort, surveys of the scientific literature or descriptions in secondary sources may turn up information regarding where species can be found in the wild. However, with careful searching, this may become an even more frustrating experience because of the great range of many coral species. For example, Stylophora pistillata or Pocillopora damicornis can exist in
widely divergent areas, from murky shallow water, to deep reef slopes, to near the surface of the water in wave-pounded areas. Acropora spp. can be equally difficult; different species are found occupying virtually every niche environment of tropical Indo-Pacific reefs. This is coupled with the fact that aquarists have little or no idea of the area of actual specimen collection, or even the country of collection. A further hindrance is the fact that there is remarkably little information available as to the range, environment, or life history of some of the most popular aquarium corals. This article, based on the events described below, is a first step in answering some of these frustrations.
“As the world’s largest importer of coral reef organisms for curios, jewelry, and aquariums, the United States has become concerned that the demand for these organisms may be a major force driving overexploitation and destructive collection practices. As one step to address
these concerns, the U.S. sponsored the International Coral Trade Workshop in Jakarta, Indonesia in April 2001 to develop recommendations for the sustainable harvest of stony corals. The workshop brought together over 130 experts from Southeast Asia, the South Pacific, Australia, Europe and the U.S., and included government representatives (Ministries
of Fisheries, Trade, Environment and CITES agencies), NGOs (TRAFFIC, WWF, IMA and MAC), scientists, coral collectors and exporters (Bruckner, 2001).” I was invited to act as co-chair for the Collection Working Group at this workshop with Ferdinand Cruz of the International Marine Life Alliance. Following the workshop, an assessment team was assembled to test the monitoring approach from the workshop. Members included coral reef biologists, fisheries biologists, industry representatives, coral collectors and students. Ed Lovell (coral taxonomist), Andrew Bruckner (coral reef ecologist), Suharsono (Indonesian coral reef scientist), and I conducted belt transects and manta tows to map species richness, distribution, abundance, population dynamics and habitat requirements of key scleractinian corals in the aquarium trade. Other divers who provided video transects and non-transect support were John Fields (NOAA Fisheries Biologist), Caroline Raymakers (TRAFFIC
Europe), and several Indonesian biologists and students. Twelve areas were surveyed in the Spermonde Archipelago of South Sulawesi, Indonesia. This effort was largely to provide scientific data to assess the ban of certain coral taxa in the European Union.
A Bit of Background
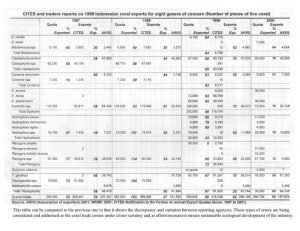
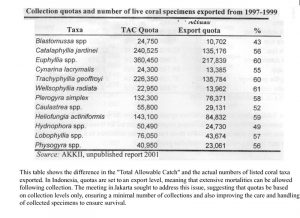
Indonesia is the world’s largest exporter of live corals for the aquarium trade. Harvest of dead or curio corals, as skeletons, was banned in 1998. A coral exporting group, the Asosiasi Kerang, Koral dan Ikan Hias Indonesia (AKKII) is the only group allowed to legally export corals. A quota and management system is in place to act as guidelines for sustainable
management of their coral reef resource (Table 3). Coral harvest, rotated among 10 provinces over four years, is prohibited in protected and tourist areas, has size limits, is supposed to occur where assessment of monitoring of the resource occurs, and is supposed to occur at
levels below regeneration rates. However, there is question as to the efficacy of this plan. The quota for 2001 included 925,000 live corals, 950,000 pieces of substrate with soft corals or other invertebrates attached, and 450 metric tons of live rock (Table 4).
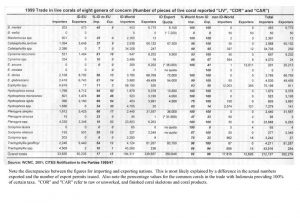
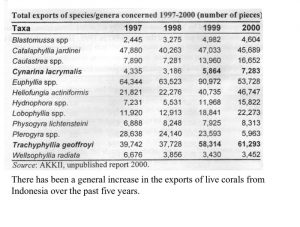
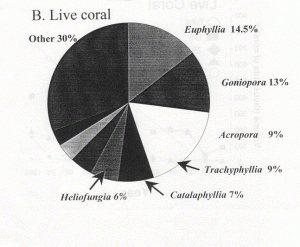
Indonesia is somewhat unique in providing aquarium corals in that the country seems to “specialize” in providing the vast majority of popular large-polyped genera. These same genera are also the ones that account for the majority of genera in the trade, by far (Graph 1). There are no species given in these figures, only genera. Included are some genera generally acknowledged to have poor survival in aquariums as well as some thought to be uncommon and slow growing with low rates of recruitment. Hence, there is concern that there may be environmental impact from their harvest. Another aspect of collection is that, despite the vast area of Indonesian coral reefs, relatively few areas are harvested. One reason for this is the
requirement that collections take place near to an airport or facility that can adequately handle and transport collected animals. As a result, the majority of collection occurs on Java (near Jakarta in the “Thousand Islands” archipelago), several sites around Bali, and sites near Makassar (Ujung Pandang) and Kendari, Sulawesi. The sites near Jakarta, with a population of over 9 million people, have long been highly over fished and degraded. Bomb fishing is rampant, and many of the reefs of this area are virtually devoid of life. Coral collection does not occur at nearly the degree that it does in Bali or Sulawesi. In fact, the areas we surveyed in the Spermonde Archipelago are part of one of the largest coral reef areas of Indonesia and one of the largest coral collection areas. They are also impacted by bomb fishing, cyanide fishing (for the aquarium trade and the live reef fish food trade), and are adjacent to a large population city that impacts near-shore areas with significant amounts of terrestrial runoff.
We conducted surveys and transects at sites where coral collectors were working, or sites described by collectors of AKKII members where coral collection takes place (Figures 2&3). They ranged from nearshore reefs from 1-10 m in depth, to patch and fringing reefs 3-20m in depth, to submerged reefs, and algal flats and mud/sand flats 23-40m in depth (Table 5). Collectors worked alone but with 4-6 divers per boat, primarily using a hookah, and regularly made long and repetitive dives to 40+m depth without using dive tables or decompression stops.

Figure 5. Coral collectors feeding hookah line to the diver on the deep seafloor collecting Trachphyllia.

Figure 4. The Spermonde Archipelago is a vast region of some 16,000 square kilometers of ocean pnctuated by small islands. Coral collection takes place not only on fringing reefs surrounding the islands, but alson on submerged reefs and the seafloor between islands.
On deep dive sites, collectors were mostly interested in collecting valuable sea cucumbers for the trepang food trade, with coral collection an incidental part of their work. However, these areas are the prime collection areas for many of the aquarium corals discussed here, but the income generated (approximately $ 0.05 apiece) was mainly used to buy cigarettes (pers comm, coral collectors). Divers used small hammers and chisels to extract corals, working quickly and spending only a few minutes finding and removing a specimen (Table 6). Corals are typically amassed at a central area on the bottom, and then transferred up to the boat quickly using a basket and rope. They are then sorted on the boat out of water and in direct sunlight before being placed into coolers or areas in the boat filled with seawater and separated by a piece of plastic film.
It should be recognized that many hours might pass at some of these remote locations before corals are put into a holding facility. While we were at one site, we were informed that the collectors had just received an order for 5,000 Cynarina sp. corals and the order had to be filled quickly. This meant intensive hunting for colonies and long hours underwater that would certainly jeopardize the health of the divers.
The local holding facility of a large exporter, CV Dinar, was observed on Baranglompo, an island over 10 km from Makassar. Here, corals are stored in slow-flowing seawater tanks for a few days to a few weeks before being boxed and shipped for transport to the exporting facility in Jakarta (Table 7). From there, they spend an additional few days to weeks at that facility (this facility lacks flowing seawater systems) until they are sent overseas or to domestic markets. To the credit of CV Dinar, the facility was well run for a developing nation, and corals seemed to be in mostly good condition. There were exceptions, however, and the overall good health of the corals may be due to their recent removal from the sea and/or their inherent hardiness.

Figure 6. These collections of Cynarina lacrymalis and Trachyphyllia geoffroyi were photographed on the deck of the collectors boat.

Figure 7. This Blastomussa colony has just been collected and sits on board the small boat until it reaches the holding facility.

Figure 11. The inshore reefs of the Spermonde Archipelago lie off the southwestern coast of Sulawesi. This is a prime coral collecting area since it is very close to the airport at Makassar (Ujung Pandang).

Figure 12. Despite having clearer waters in more remote areas of the archipelago, this turbid near shore reef has a very high coral coverage but the water is very dark, being rich in shore-based sediments.
What We Found
To say that this study was enlightening would be an understatement. While the results of the transects were presented to some degree at MACNA XIII (Baltimore) by Dr. Bruckner, and will be covered in an upcoming paper we will produce as soon as all the data is amassed and studied, my purpose in writing here is to provide the fascinating accounts of where many of our most popular aquarium corals are found. This is some of the first information ever provided regarding their location and habitat, and it is my hope that finally aquarists will have some idea of the conditions that may be most appropriate for these corals in aquariums, ensuring their success and survival.
I can only hope that our future surveys and the guidelines being developed will provide even more information, and that information promised to be gathered by the Marine Aquarium Council (MAC) and the exporting nations/collectors can provide more information to help benefit aquarist efforts in coral care.
Trachyphyllia geoffroyi (Open Brain Coral)
(Table 8). There are two major collection habitats for Trachyphyllia. The coral known as Wellsophyllia is also reported on Indonesia quotas, but Wellsophyllia is actually synonymous with (or possibly a separate species/subspecies of) Trachyphyllia. Trachyphyllia were found mostly as unattached, free-living colonies, although in one habitat they were frequently found attached to reef substrate along with unattached colonies.
Habitat 1: Trachyphyllia, such as those most commonly seen in US markets as the brilliant green, red, or pink morphs, are collected far offshore in deep waters from 30-40m in depth. They are found on extensive soft bottom areas between islands (often with no land in sight), spaced sporadically across the seafloor. The sediments are fine sands and silt, and the area is covered with cyanobacterial mats. Macroalgae is common, rooted in the substrate. There is no coral reef present, but other solitary or free-living corals are found here. Because of the habitat and ease of collection Goniopora, Cynarina, Catalaphyllia, Sinularia, Herpolitha, small Euphyllia, and a few other genera can be gathered here. However, this does not appear to be a primary collection area for any corals except Catalaphyllia, Cynarina and Trachyphyllia. The current at the bottom is very slow, but present, and probably reflects tides more than any other source. The water in the archipelago is not crystal clear, and at this depth, the water was quite dark and light levels were very low. I wouldn’t expect many zoooxanthellate corals to be able to survive with such low irradiance.

Figure 18. These drab colored and mottled Trachyphyllia are found in near shore areas (pictured above) in shallow water with very high silt levels.
Habitat 2: The other area where Trachyphyllia are collected is quite different and consisting of shallow areas from 3-4m in depth. Here, Trachyphyllia are quite abundant, but rare any deeper. It is an area that is very near shore, and runoff and river flow leaves the water with a visibility of 2-5m maximum. The reef has high levels of sediment, and Trachyphyllia are found in both attached and unattached forms, nearly buried in silt. The coral coverage of these reefs was the highest we recorded, and was nearly 100% covered by stony corals, with Galaxea and Montipora being by far the most abundant taxa present. The area is also very extensive, and it does not appear that there is any likelihood of over collection in these areas On several dives, I could not see the bottom until my mask was nearly pressed into the sand and silt. Catalaphyllia is also reported to be collected here, and is supposedly one of their primary collection areas (see below). Currents were low and light levels reduced because of the amount of suspended silt in the water column. However, this may vary depending on the season or conditions over time. Nonetheless, it is somewhat irrelevant; the Trachyphyllia that are found here are not the morph most popular in the trade. They are drab in color; brownish pink or brownish green and somewhat mottled or striped. We did see these morphs in the exporting facility, but they were not common, and they were rarely seen being held at the collection facility. I have also seen these morphs in US aquarium stores, but again, they are not common.
Euphyllia spp. (Anchor, Frogspawn, Hammer and Torch corals)
Euphyllia spp. have been listed by leading coral scientists as being uncommon (Veron 1986, 2000). This
designation was considered in the EU’s temporary ban of selected species. We were quite surprised to find that, at least in the Spermonde Archipelago, this was not the case. Euphyllia species were found at all reef sites, and at some sites in high abundance. Although most of the colonies were small, there were occasions when we saw very large colonies. We also saw most, if not all of the species known, including E. glabrescens, E paraglabrescens, E. cristata, E. yaeyaemaensis, E. ancora, E. parancora, E. divisa, and E. paradivisa. Only E. cristata was uncommon in our transect. The primary collection areas for Euphyllia are patch and fringing reefs from 4-25m in depth. These are mostly clear water sites, although clear water in the Spermonde is still somewhat turbid and greenish. Currents vary with conditions, but were quite calm during our dives. Surprisingly, I would estimate that half or more of the colonies were of branching species, considering they are less common in the trade. Deepwater sites varied in their abundance of Euphyllia, but deeper sites (>30m) contained almost exclusively small colonies of the branching species. Their skeletons are subject to much bioerosion, and I don’t think colonies can get very large as a result. Furthermore, at a few deepwater sites, there is no hard substrate for them to form secure attachments above the sediment surface, resulting in smallish colonies. Unfortunately, there were no other consistent morphological or skeletal differences to distinguish colonies between the habitats, and therefore specimens of Euphyllia found in an aquarium stores may be from very different habitats and subject to very different conditions (Table 9).
These Euphyllia are just some of the species photographed across a range of habitats in the Spermonde Archipelago:
Catalaphyllia jardinei (Elegance coral)
Habitat 1: Perhaps one of the bigger surprises came from our attempts to locate Catalaphyllia. We were assured that Catalaphyllia would be very common and collected from several sites. In fact, it was quite uncommon at all sites and the locations given by coral collectors seemed to vastly overstate its abundance. Fortunately, we did find examples at several different locations and, like Euphyllia, in very different habitats. Unlike Euphyllia, we found them to adopt quite distinct morphotypes, depending on the area of collection. The deepwater sites where Trachyphyllia were collected were also the area where we saw the most harvest of Catalaphyllia. To repeat, this is a very low-light sand and silt seafloor with no hard substrate and colonized by sparsely populated free-living corals, macroalgae, and cyanobacterial mats. Here, Catalaphyllia were found as small, free-living colonies that are generally the size and shape of the vast majority seen in aquarium stores. However, every coral seen – and every Catalaphyllia collected – from this area had purple tentacle tips. None of the Catalaphyllia at any other site had this characteristic.
Habitat 2: In contrast, the Catalaphyllia we found (rarely) at fringing, patch, and shallow submerged sites were mostly medium to large attached colonies. These colonies grew on the hard reef substrate and seemed to be able to grow much larger, adopting significantly developed flabello-meandroid growth forms. Furthermore, their skeleton, being attached, would be broken off for collection, rather than having the cone-shaped or unbroken bases typical of free-living colonies at all other sites. The colonies had the typically seen color patterns of bright green to brown with radiating stripes on the oral-disk, and brownish tentacles. Furthermore, the skeletons were well cleaned and whitish from grazing, and coralline algae and other typical invertebrates were found colonizing the skeleton. This is notable, given the next habitat description.
Habitat 3: The same general near shore, silty, shallow area where the dull colored Trachyphyllia were collected is also reported to be a prime area of Catalaphyllia collection. We did find a few specimens using searching and manta-tow techniques, but none ever appeared on any transects of the area. Therefore, we must assume a sporadic occurrence of lowdensity. Like the deepwater habitat, Catalaphyllia collected here were free-living and never attached. They were found nearly buried in deep fine silts and were, also like the deepwater colonies, small and apparently size-limited by the substrate. More notably, the thick brown silt had discolored their skeletons from white to a dingy brown. The only notable growth on the skeleton was from the calcified tubes of polychaete worms. Their coloration was drabber, being brownish with muted green-brown oral disks and tentacles.

Figure 25. Catalaphyllia jardinei, displaying the much sought-after purple tentacle tips, are photographed here between 30 and 35m in depth. Note the silt deposits on the colony shown.

Figure 26. Catalaphyllia are displayed in a flow-through tank at the Baranglompo holding facility. Note the highly distended oral disks resulting from extremely low water flow. This is not, however, the same as the Catalaphyllia “condition” shown below.

Figure 27. Catalaphyllia are displayed at an exporter in Jakarta. The colony in the middle is displaying the condition that characterizes many Catalaphyllia being imported to the US in recent years. The swollen and discolored oral disk with a shriveled fringe of tentacles is a condition from which few survive. The other colonies display a normal appearance. We did not observe this condition underwater, but the short period of time between their collection and their arrival here, given our observations of the entire collection process, makes it likely that the corals are indeed afflicted in the wild and not as a result of some collection or shipping stress. A parasitic gall crab has recently been found beneath the tissue of almost all Catalaphyllia examined with this condition (Shimek pers comm, www.rshimek.com).
Cynarina lacrymalis (Button or Meat coral)
Even the coral collectors admitted, that Cynarina were significantly difficult to find .In fact, they couldn’t point out any areas where one could just go and find them readily; searching an area – and subsequent collection wherever one was found – seemed to be the norm for them. We also found this to be true in our many dives, and transects showed the coral to be extremely rare to absent at all sites. However, they were present at patch, fringing and submerged reef habitats, and in the deepwater habitat as free-living colonies. Most were very small juveniles, but there was one very large specimen and several of average collected size. Like Euphyllia, it is hard to generalize on the preferred habitat. If anywhere, the density was highest (found by searching but not by transect) in the 30-40m algal flat deepwater habitats. On the reef, Cynarina was found occasionally; always attached and never free-living. Once again, the skeleton will provide clues as to whether the coral was attached or free-living, and may therefore be used to approximate the likely collection habitat. Attached Cynarina were always found lodged into nooks and crannies of the reef, under overhangs, and under corals. They appear to prefer or dwell almost exclusively in extremely protected areas receiving very low light levels. There were no other determining morphological characters we found that could be used in assessing habitat.

Figure 28. Collectors preferentially target free-living Cynarina lacrymalis found in deepwater, such as this one, because they are easily collected and are found along with other corals sought for the trade.

Figure 29. Collectors preferentially target free-living Cynarina lacrymalis found in deepwater, such as this one, because they are easily collected and are found along with other corals sought for the trade.

Figure 30. The attached form of Cynarina lacrymalis was also found in transects on patch and submerged reefs. It was uncommon, and almost always found like this one; in shaded and protected niches within the reef framework.

Figure 31. Unlike its relative Cynarina, Scolymia, while not abundant, was much more common in coral collecting areas. It was always found as solitary attached colonies on patch, fringing, and submerged reef slopes.
Nemenzophyllia turbida (Fox coral)
I must say that finding and seeing Nemenzophyllia turbida in the wild was one of the most interesting discoveries and a high point in my diving career. This coral was only first described in 1981 (Hodgson and Ross 1981) and was questioned as being a true species for over a decade more (Veron 1986, pers comm.). There is almost nothing known of its abundance, range, or habitat. The collectors described Nemenzophyllia as terribly common and, where we found it, it was unquestionably common. However, Nemenzophyllia seems to have locally abundant but patchy distribution. Its true abundance remains unknown, but could be either very great or very limited.
Where is Fox coral found and collected? It is found in vast dense tracts on the seafloor bottom 33-35m down, mixed equally with free-living Goniopora spp. Other corals in smaller numbers consist of Alveopora, Sarcophyton, zoanthids, Sinularia, Euphyllia, and a possibly new species of Lobophyllia. Nemenzophyllia turbida is found in fields, sitting on the bottom with polyps facing upward. The colonies are mostly broken apart, and they are perhaps asexually populating these fields by bioerosion-induced fragmentation as no buds were seen on colonies. The entire area is bathed in silty deposits and sits perhaps a half-meter above the surrounding seafloor, a platform composed entirely of the dead skeletons of corals found there as silt and bioeroding organisms bury and erode previous growth. The corals are all free-living, none are attached. Goniopora and Nemenzophyllia exist in constant contact with each other, with no notable competition occurring. Almost every colony of coral is touching every adjacent colony. In places, Nemenzophyllia abundance became lower, leaving mostly all Goniopora on the bottom. We are not sure if this is a natural patchy distribution or the result of harvest. It appeared to be the latter The water flow at this depth was very low, and the area, although nearer to small islands and closer to shore than the deepwater Trachyphyllia site, was still between islands in the channel. The light levels were very low, and some very random occasional bleaching was occurring in some colonies of several genera – we are unsure why this might be happening, given the temperature and the light available. Also found here was the very similar looking and recently described Plerogyra discus (Veron 2000). It was not common, and was found blending almost perfectly with Nemenzophyllia. We also saw this coral being collected and sold, not surprisingly, as Fox coral.

Figure 34. Plerogyra discus is almost impossible to tell from Nemenzophyllia turbida at a glance. However, the difference is apparent here, with polyps arising from individual skeletal elements. We found this coral intermixed with Nemenzophyllia in the same area.

Figure 35. Here, the much more abundant Nemenzophyllia turbida is seen in the front-center and back-right, with Plerogyra discus at the left-center. Without picking the colonies up, they look almost indistinguishable while diving, and it was by chance that I discovered its existence here.
Blastomussa spp. (Swollen or Closed Brain coral)
Blastomussa is comprised of two species, B. merleti and B. wellsi; both collected for the aquarium trade primarily from Indonesian waters. Like Cynarina, collectors harvest Blastomussa wherever they can find it using searching techniques, and they acknowledge that it can be hard to find. It was one of the rarest corals in our many transects and only one or two were found on any given transect; at many sites there were none found, even by searching. They appear to be found on the deeper part of the reef slope on fringing reefs and on deeper submerged patch reefs. Blastomussa are found in small colonies, usually no more than a dozen polyps per colony, more typically three to five. They were found partly to almost completely protected from any direct light and often occupied vertical positions. All recorded colonies were B. wellsi, and only a single colony of B. merleti was found at all. Perhaps more surprisingly is that there is no quota in Indonesia for B. wellsi, only for B. merleti, and yet no colonies of that species were found or seen at collection or exporting facilities. There were no particular characteristics that could be used to delineate the habitat where these colonies were found except that they seem to prefer sheltered deeper locations, as has been reported elsewhere for the genus.

Figure 36. This small colony of Blastomussa wellsi is typical of what we found in transects; very rare and found in extremely isolated colonies of a few polyps each in most habitats except the deep algal flats.

Figure 37. A large Blastomussawellsi colony; an extremely unusual find. This colony did not appear within our transects but was found by chance during a dive on a submerged patch reef slope.

Figure 38. This typical colony of Blastomussa wellsi has just been collected from a low relief deep site.
Others, Briefly
Plerogyra spp. We found Plerogyra in most habitats, mostly on the fringing and patch reef slopes. Colonies were typically quite small and aquarium sized. They were fairly common, and both the normal and “octobubble morph” were present. Also present, but much less common was the branching Plerogyra simplex. Like Blastomussa, they were mostly oriented on vertical surfaces (Table 10).

Figure 39. The most common form of Plerogyra sinuosa. We found most colonies to be small and “aquarium-sized,” although larger colonies like this one were not uncommon.

Figure 40. The branching Plerogyra simplex; this species was not common in any area, although not rare, either.
Goniopora spp./Alveopora spp./Fungia spp. What can I say about Goniopora? It’s still wrong, in my opinion, to be taking so many of these corals from the wild only to have such a terribly high percentage of them die in aquariums. But, they are everywhere in the Spermonde archipelago: in massive droves, in monospecific fields, and not in monospecific fields. However, this is not the case in many areas of Indonesia and Sulawesi. If anything, the conditions of Spermonde again point to aquarium-collected Goniopora thriving in high nutrient
and turbid waters. Alveopora were similar in their habitat range, occurring most commonly in the Nemenzophyllia area, but were much less common in all areas.
Perhaps the only genus more common than Goniopora on most of these reefs was Fungia, a genus only conspicuously absent from the deepwater algal flat areas and the deep channel where Nemenzophyllia was found. The many species of Fungia were not identified although dozens were present and in abundance that placed several dozen within a square meter of some transect areas. Guidelines such as those given in Aquarium Corals (Borneman 2000) may give a better guide to conditions they require.

Figure 42. Red Goniopora are reported to be collected almost exclusively from the Makassar area, and although obviously collected (as shown here at the holding facility in Baranglompo), we did not find these corals in our transects, nor did we learn of the site where they are collected.

Figure 43. This Alveopora is photographed alongside the much more abundant free-living Goniopora, on the “psuedo-hardbottom” created by silt-smothered, loosely deposited, dead coral skeletons in the Nemenzophyllia collecting area.

Figure 44. Strangely, we found numerous cases of a solitary bleached Goniopora among fields of the same species Goniopora. This specimen is seen next to normally pigmented Goniopora and Nemenzophyllia. Considering the water clarity was not high and we were more than 100 feet below the surface, the cause of the bleaching was quite mysterious.
Montipora spp. – While occurring on the patch, fringing and submerged shallow to mid-depth reefs, most of the species of Montipora commonly seen in the aquarium trade were found in very high abundance in the nearshore areas where the drab Trachyphyllia were found. The most common species were the foliaceous and turbinate forms usually sold collectively (and often incorrectly) as Montipora capricornis. However, Indonesians are not prone to collecting small-polyped corals, despite their ready availability and great abundance throughout the country.
Galaxea spp. – Possibly the third most common coral genus found at most of the fringing, patch and submerged mid-depth reefs was Galaxea. At the nearshore site where the drab Trachyphyllia were found (see photos above), Galaxea was the dominant genus, and formed expansive coverage, often covering nearly 100% of large areas. It seems to thrive most in these muddy, silty waters, although it was also
common and did occur in smaller colonies in clearer water.

Figure 45. Caulastrea was very uncommon at all sites, and yet they are under heavy collection pressure because of demand. This beautiful colony is seen with Zoanthus sp. between the corallites at an exporter in Jakarta.

Figure 46. Heliofungia actiniformis is under heavy harvest pressure because of demand. Fortunately, they were quite common in many areas since it has a relatively poor record of survival in aquariums.
Summary
By now, I have done some fairly extensive diving in Indonesia and have visited some very diverse reef types and sites during several trips. I have seen some of the most pristine off shore areas of clearwater and some of the muckiest, silt-filled areas. Without question, the abundance and morphotypes I have described here do not fit all the sites where these corals could be found, nor does it describe their range. However, for aquarists, I have seen the areas where large numbers of corals collected for the aquarium trade are harvested. It doesn’t matter so much that one might find these species in the Moluccas far offshore, or perhaps in the Komodos. Corals are not being collected there. Corals are collected from limited sites of a similar nature, near to airports. Here, in the Spermonde Archipelago, the characteristics given are true for the sites where corals are being collected, and for those corals that end up in the aquarium trade. Future surveys at different sites may prove that there is more of the story that needs to be told. For now, though, I hope this information is just the beginning of the type of information needed by aquarists in order to replicate habitat conditions for some of the popular corals they are keeping, and that such information can be used to increase survival in aquariums and reduce impact on wild coral reefs in the area. The additional appendices at the end of this paper will provide summary data for this work.
Acknowledgements:
Thanks to Andrew Bruckner, Caroline Raymakers, Ed Lovell, Suharsono, John Fields and the other participants of the workshop in Jakarta and the assessment team. Also thanks to NOAA/NMFS, AKKII, and the other sponsors who made this work possible.
References and Literature Used
- Borneman, Eric. 2001. Aquarium Corals: Selection, Husbandry and Natural History. TFH Publications/Microcosm, Ltd., neptune City, NJ. 464 pp.
- Bruckner, A.J. 2001 Proceedings of the International Workshop on the Trade in Stony Corals: Development of Sustainable Management Guidelines April 9-12 2001 Jakarta, Indonesia.
- Hodgson, Gregor and Michael A. Ross. 1981. “Unreported scleractinian corals from the Philippines.” Proc 4th Int Coral Reef Symp, Manila 2: 171-175.
- Raymakers Caroline. 2001. “Review of trade in live corals from Indonesia.” TRAFFIC Europe, Brussels. 98pp.
- Veron, J.E.N. 2000. Corals of the World Vol 2. AIMS, Townsville: 86-87.
- Veron, J.E.N. 1986. Corals of Australia and the Indo-Pacific. University of Hawaii Press, Honolulu, Hawaii. 644 pp.
- For a photo of the parasitic gall crab of Catalaphyllia, visit www.rshimek.com



















0 Comments