In previous articles I have dealt in detail with issues involving magnesium1 and strontium2 in reef aquaria. While information on the amount of strontium and magnesium delivered by calcium carbonate/carbon dioxide reactors has been previously shown, similar information has been unavailable for limewater.
This article gives experimental data on the amount of magnesium and strontium present in one commercial source of calcium oxide (Mississippi Lime Company; Figure 1) that many aquarists have found to be an inexpensive source of high quality lime (although it must be bought in very large lots from distributors). It also shows how those ions might or might not actually make it into the water column of reef aquaria, and compares the amount of these ions delivered by limewater to the amount used by corals in depositing their skeletons. Because of a potential imbalance between the amount of magnesium and strontium used by corals, and the amount delivered by limewater, using limewater can potentially lead to depletion of both ions in aquaria.
In the past, there has been significant discussion of the depletion of magnesium by limewater. Craig Bingman3 showed that precipitation of magnesium carbonate and hydroxide in aquaria using limewater was not very likely to be significant. More likely, such depletion is simply the result of not delivering as much magnesium to the aquarium as is being “exported” during calcification. This article shows how this depletion might work for both magnesium and strontium.
How Much Magnesium Do Corals Consume?
The amount of magnesium incorporated into the skeletons of various calcifying organisms varies considerably. In a previous article,1 I showed that corals in the ocean can incorporate between about 0.1% and 3.5% by weight magnesium in their skeletons. Coralline algae also incorporate a considerable amount, typically more than 1%, and as high as 4.4% by weight. There are few data on coral skeletons in aquaria, but the magnesium content is not expected to be significantly different from this range.
Calcium is present in these skeletons at about 35-38% by weight, since they are largely calcium carbonate. Consequently, the Mg/Ca ratio ranges from about 0.0025 to 0.12 by weight (on a mole basis, these Mg/Ca ratios are about 0.004 to 0.2; all subsequent Mg/Ca ratios in this article are by weight).
Consequently, if one wanted a calcium supplement to be the sole source of magnesium in an aquarium, then it would have to include approximately this same Mg/Ca ratio (0.0025 to 0.12) to preclude building up or depleting magnesium over time. Obviously, with such a wide range, the exact balance in any given aquaria will be determined in part by the mix of corals and coralline algae being maintained. Fortunately, there is such a large reservoir of magnesium in seawater that it takes large differences between import and export to cause important changes in magnesium levels.
How Much Strontium Do Corals Consume?
The amount of strontium incorporated into the skeletons of various calcifying organisms does not appear to vary as much as magnesium. In a previous article,2 I showed that corals typically incorporate about 0.9% by weight strontium in their skeletons.
Calcium is present in these skeletons at about 35-38% by weight, since they are largely calcium carbonate. Consequently, the Sr/Ca ratio is about 0.02 by weight (this Sr/Ca ratio is about 0.01 on a mole basis; all subsequent Sr/Ca ratios in this article are by weight).
Consequently, if one wanted a calcium supplement to be the sole source of strontium in an aquarium, then it would have to include approximately this same Sr/Ca ratio (0.02) to preclude building up or depleting strontium over time. Unlike magnesium, there is not much strontium present in seawater. Consequently, it does not take a large imbalance between import and export to significantly skew strontium levels in aquaria.
What is limewater?
Limewater4,5 (also known by the German term kalkwasser) has been used very successfully by aquarists for a number of years, and it is the system that I use on my reef aquarium. It is comprised of an aqueous solution of calcium and hydroxide ions that can be made by dissolving either quicklime (calcium oxide, CaO) or lime (calcium hydroxide, Ca(OH)2). The only inherent difference between the two is that if you add a molecule of water to quicklime, you get lime, and that a great quantity of heat can be generated when that happens.
CaO + H2O → Ca(OH)2
Quicklime + Water → Lime
Consequently, hydrating and dissolving quicklime can make water quite warm, especially if an excess of solids are added. One aquarist contacted me after he melted his limewater reactor by putting quicklime into it. So understanding the difference between the two forms does have advantages!
The calcium ions in the solution obviously supply calcium to the tank, and the hydroxide ions supply alkalinity. Hydroxide itself provides alkalinity (both by definition6 and as measured with an alkalinity test), but corals consume alkalinity as bicarbonate7, not hydroxide. Fortunately, when limewater is used in a reef tank, it quickly combines with atmospheric and in- tank CO2 and bicarbonate to form bicarbonate and carbonate:
OH– + CO2 → HCO3–
OH– + HCO3– → CO32- + H2O
Once in the tank at an acceptable pH,8 there is no concern that the alkalinity provided by limewater is any different than any other carbonate alkalinity supplement. The hydroxide immediately disappears into the bicarbonate/carbonate system. In other words, the amount of hydroxide present in tank water is really only a function of pH (regardless of what has been added), and at any pH below 9, it is an insignificant factor in alkalinity tests (much less than 0.1 meq/L). Consequently, the fact that alkalinity is initially supplied as hydroxide in not to be viewed as problematic, except as it impacts pH (see below).
The fact that limewater is very basic (the pH is typically above 12) demands that the limewater be added slowly to a tank unless very small additions are made. The reason for slow addition is two-fold: to prevent the local pH in the area of the addition from rising too high (slow addition permits more rapid mixing with tank water to reduce the pH), and to prevent the overall tank pH from rising too high (slow addition allows the tank to pull in CO2 from the atmosphere during the slow addition, mitigating the pH rise). Some aquarists advocate rapid addition,9 and that is fine for additions that would add less than 0.2 meq/L of alkalinity to the tank, but an addition of 0.5 meq/L (the equivalent of adding 1.2 % of the tank volume in saturated limewater or 14 grams of calcium hydroxide into a 100-gallon tank) drives the pH of the whole tank too high (up by about 0.5 pH units).10
Consequently, limewater is most often added slowly, by dripping or slow pumping. Often it is added as the top off water, replacing most or all of the evaporated water. The pumps add cost and complexity to the system, especially if combined with a float valve or switch (I use the latter and a Reef Filler pump).
The high pH of limewater can also have significant advantages with respect to impurities present in the lime. Phosphate and many heavy metals will precipitate, either as calcium salts, or as metal oxides and hydroxides. Copper, for example, has been suggested by Ron Shimek to be a concern in reef aquaria.11, 12 Copper hydroxide is very insoluble in limewater, and settled limewater can have fewer impurities than the water or lime used to make it.13
It also turns out that magnesium is very insoluble at high pH. In limewater, its low solubility can result in precipitation of magnesium hydroxide on the bottom of a limewater reservoir. This precipitation limits how much of the magnesium in the lime is delivered to aquaria, as will be shown below.
What is in Lime?
The calcium oxide that I use from the Mississippi Lime Company is food grade (Figure 1). The company lists a number of impurities in it, but does not break out magnesium and strontium. Table 1 shows a typical analysis supplied by the manufacturer. Table 2 shows the required limits to food grade CaO.
| Component | Percent |
|---|---|
| Si | 0.35% |
| CaO | 98.0% |
| LOI | 0.50% |
| Magnesium & Alkali Salts | 1.0% |
| Fluoride | 75 ppm |
| Lead | <0.5 ppm |
| Arsenic | <1.0 ppm |
| Acid Insoluble Substances | 0.20% |
| Heavy Metals | 2 ppm |
| Al | 0.10% |
| Fe | 0.04% |
| S | 0.01% |
| CO2 | 0.40% |
| P | 50 ppm |
| Mn | 12 ppm |
| Ca | 69.97% |
| Crystalline Silica | <0.1 |
| Item | Specification |
|---|---|
| CaO by assay | 95.0 to 100.5% |
| Loss on Ignition | Not more than 10% |
| Magnesium and Alkali salts | < 3.6% |
| Fluoride | < 150 ppm |
| Lead | < 5 ppm |
| Arsenic | < 3 ppm |
| Acid Insoluble Substances | < 1% |
| Heavy Metals | < 30 ppm |
The only information provided about magnesium that “Magnesium and Alkali salts” are ~1 % by weight. In this context, alkali salts can include sodium, potassium, and lithium. Consequently, it is not a very useful number for understanding how much magnesium is present. Lime can actually come with a whole range of possible magnesium concentrations. According to the National Lime Association:
“Quicklime, the product of calcination of limestone, consists of the oxides of calcium and magnesium, and in the United States it is available in three forms:
- High calcium quicklime –derived from limestone containing 0 to 5 percent magnesium carbonate.
- Magnesian quicklime–derived from limestone containing 5 to 35 percent magnesium carbonate.
- Dolomitic quicklime–derived from limestone containing 35 to 46 percent magnesium carbonate.
Hydrated lime is a dry powder manufactured by treating quicklime with sufficient water to satisfy its chemical affinity for water, thereby converting the oxides to hydroxides. Depending upon the type of quicklime used and the hydrating conditions employed, the amount of water in chemical combination varies, as follows:
- High calcium hydrated lime–high calcium quicklime produces a hydrated lime containing generally 72 to 74 percent calcium oxide and 23 to 24 percent chemically combined water.
- Dolomitic hydrated lime (normal)–under atmospheric hydrating conditions only the calcium oxide fraction of dolomitic quicklime hydrates, producing a hydrated lime of the following chemical composition: 46 to 48 percent calcium oxide, 33 to 34 percent magnesium oxide, and 15 to 17 percent chemically combined water.
- Dolomitic hydrated lime (pressure)–this lime is produced from dolomitic quicklime under pressure, which results in hydrating all of the magnesium oxide as well as all of the calcium oxide, producing the following chemical composition: 40 to 42 percent calcium oxide, 29 to 30 percent magnesium oxide, and 25 to 27 percent chemically combined water.”
There is no information provided by either the Mississippi Lime Company, or the National Lime Association on the amount of strontium in any of these products.
Experimental Results: What Is Present In Quicklime?
In order to better assess how much strontium and magnesium would be delivered by the use of this brand of lime, I determined how much calcium, magnesium, and strontium were present using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES). Details of the method are given at the end of the article for those who may want to reproduce it on other materials. The results are shown in Table 3.
| Metal | Absolute Concentration in solid (weight %) | Relative Concentration (by weight) |
|---|---|---|
| Magnesium | 0.25% | 0.0038 = Mg/Ca ratio |
| Calcium | 65.5% | 1.00 |
| Strontium | 0.024% | 0.00037 = Sr/Ca ratio |
As expected, the primary ingredient is calcium. Magnesium is fairly low, and strontium is quite low. This material has a Mg/Ca ratio of 0.0038. That is at the low end of the Mg/Ca ratio found in corals, and well below that found in coralline algae. It has a Sr/Ca ratio of 0.00037. That Sr/Ca value is far below the 0.02 ratio for Sr/Ca found in typical corals.
Experimental results: what is present in limewater?
I dose my aquarium with limewater made from the quicklime tested in the previous section. I typically use limewater at less than saturation because my reef aquarium does not need full strength limewater. In order to test for magnesium and strontium in the limewater that I dose, I made 44 gallons of limewater, and dosed it for about 3 weeks. I then took a sample of the clear limewater that remained. It had a conductivity of 7 mS/cm, indicating that it is not saturated (saturated limewater usually has a conductivity of about 10.3 mS/cm).14 This sample was analyzed as is, and the results are shown in Table 4.
| Metal | Concentration (ppm) | Relative Concentration (by weight) | Enrichment Relative to Solid Lime |
|---|---|---|---|
| Magnesium | 0.017 | 0.000028 | 0.007 |
| Calcium | 610. | 1.00 | 1.00 |
| Strontium | 0.24 | 0.00039 | 1.05 |
It is interesting to note that relative to calcium, magnesium is greatly underrepresented compared to that in the starting quicklime. The reason for this result is the well known insolubility of magnesium hydroxide. Any magnesium ions released into the solution rapidly combine with hydroxide to form insoluble magnesium hydroxide that precipitates.
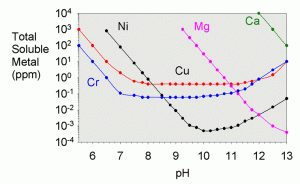
In a previous article on the solubility of metals in limewater,13 I showed a graph of the theoretical solubility of magnesium as a function of pH (below).
At the pH of limewater (low 12’s) the solubility is between 0.01 and 0.001 ppm. The experimental solubility here is a tad higher (0.017 ppm), presumably for one of two reasons: some particulates of magnesium hydroxide may have been present in the solution which are detected as soluble magnesium when in fact, it is not. A second possibility is that the solution has simply not reached thermodynamic equilibrium, and the theoretical limit to solubility has not yet been reached.. Nevertheless, the point is that it is expected that magnesium hydroxide will precipitate from such a solution, and this did, in fact, happen. The magnesium in solution was depleted by a factor of more than a hundred compared to what would have been in solution had it all been soluble. In the next section of this article, the precipitate on the bottom of the limewater reservoir was tested to show this extra magnesium.
Relative to calcium, strontium was nearly unchanged in the limewater compared to the solid quicklime. The slight elevation in the limewater is above the background noise, however, since the emission signal from strontium is so strong. The reason for this slight elevation is that strontium is even less likely than calcium to precipitate onto the bottom of the limewater reservoir, and so stays more in solution than calcium.
Experimental Results: What Is Present In Limewater Sludge?
The solids on the bottom of a limewater reservoir contain everything that did not dissolve, or that dissolved and later precipitated from solution. Such solids could contain magnesium hydroxide and carbonate, calcium hydroxide and carbonate (though calcium hydroxide is fairly unlikely in unsaturated limewater) and a variety of other impurities, such as alumina, silica, etc.
In order to determine what was in it, a sample of the white solids was taken from my limewater reservoir where it had been collecting for months. It was removed along with some limewater. The mixture of solid and liquid was acidified as was the solid quicklime (but using half as much acid since it was not as concentrated), and the constituents were determined by ICP. The results are shown in Table 5. Only relative concentrations are shown as no effort was made to dry the sample prior to analysis, making absolute concentrations meaningless.
| Metal | Relative Concentration (by weight) | Enrichment Relative to Solid Lime |
|---|---|---|
| Magnesium | 0.05 | 13 |
| Calcium | 1.00 | 1.00 |
| Strontium | 0.00019 | 0.5 |
As anticipated, relative to calcium, magnesium is enriched by a factor of 13 in the sludge compared to the solid quicklime. This magnesium may be present as both magnesium hydroxide and magnesium carbonate, but because magnesium carbonate is fairly soluble compared to calcium carbonate, it is most likely that the primary magnesium salt is magnesium hydroxide. It may also be as mixed calcium and magnesium carbonates.
Interestingly, strontium is actually depleted by a factor of 2 relative to solid quicklime, indicating that it is less likely to end up on the bottom of the reservoir than is calcium. While strontium carbonate is somewhat less soluble than calcium carbonate, the strontium concentration in the limewater is so low that SrCO3 may not actually be saturated, so the precipitation is less. The strontium that is there may simply be copreciptiated with calcium carbonate.
Models of Magnesium Depletion
In order to understand what happens over time to magnesium levels in an aquarium using this quicklime, I have developed some simple mathematical models. In the first model, we examine what effects there are if all of the magnesium in the quicklime enters the aquarium. This might happen, for example, if the quicklime is first fully acidified to pH 7 or less with vinegar (not something that I recommend). It may also happen if you deliver a slurry of quicklime (or cloudy, unsettled limewater) to the aquarium.
In this model, no magnesium is assumed to be lost due to any process except calcification, and no magnesium is assumed to be added except that coming from the quicklime. For this model we will assume that magnesium is removed from the aquarium as a coprecipitate with calcium carbonate at an average level of 1% magnesium by weight (a Mg/Ca ratio of about 0.025). An aquarium with a heavy load of organisms that use more magnesium, like coralline algae, may show a larger depletion of magnesium.
We also assume that the quicklime is added with 0.25% magnesium by weight (as was determined above). Table 6 shows what happens to magnesium over time when the aquarium is dosed with the equivalent of 0.5, 1, and 2% of the tank volume daily in saturated limewater. Even though the supplement may not be added as saturated limewater, many aquarists can readily relate to percentages of the tank volume in saturated limewater. This is just a unit of measure, and does not indicate exactly how the lime is added. Specifically, the 0.5%, 1%, and 2% levels correspond to 0.6, 1.1, and 2.3 grams of CaO per 100 liters of tank volume per day, respectively.
| Equivalent amount of quicklime added daily | Starting Magnesium (ppm) | Magnesium Added over 1 year (ppm) | Magnesium Removed over 1 year (ppm) | Final Magnesium (ppm) |
|---|---|---|---|---|
| 0.5% of tank volume | 1280 | 5 | 37 | 1248 |
| 1% of tank volume | 1280 | 10 | 74 | 1216 |
| 2% of tank volume | 1280 | 21 | 149 | 1152 |
In a second model, instead of delivering everything in the quicklime, we look at adding just what is present in clear, settled limewater. From the tests above, it contains 0.017 ppm magnesium. Since magnesium is depleted from the settled limewater, we anticipate that the magnesium depletion in the aquarium will be even more significant. The results are shown in Table 7.
| Equivalent amount of quicklime added daily | Starting Magnesium (ppm) | Magnesium Added over 1 year (ppm) | Magnesium Removed over 1 year (ppm) | Final Magnesium (ppm) |
|---|---|---|---|---|
| 0.5% of tank volume | 1280 | 0.03 | 37 | 1243 |
| 1% of tank volume | 1280 | 0.06 | 74 | 1206 |
| 2% of tank volume | 1280 | 0.12 | 149 | 1131 |
As expected, the magnesium depletion is faster when dosing the settled limewater. The difference is not all that great, however, because both the solid quicklime and the settled limewater are significantly deficient in magnesium (relative to typical corals and coralline algae).
Models Of Strontium Depletion
The same model can be applied to understand what happens over time to strontium levels in an aquarium using this quicklime. In the case of strontium, it does not matter if one lets the limewater settle or not because the strontium itself does not appreciably settle.
As in the magnesium model, no strontium is assumed to be lost due to any process except calcification, and no strontium is assumed to be added except that coming from the quicklime. For this model we will assume that strontium is removed from the aquarium as a coprecipitate with calcium carbonate at an average level of 0.9% strontium by weight.
We also assume that the quicklime is added with 0.024% strontium by weight (as was determined above). Table 8 shows what happens to strontium over time when the aquarium is dosed with the equivalent of 0.5, 1, and 2% of the tank volume daily in saturated limewater (0.6, 1.1, and 2.3 grams of CaO per 100 liters of tank volume per day, respectively)
| Equivalent Amount Of Saturated Limewater Added Daily | Starting Strontium (ppm) | Strontium Added Over 1 Year (ppm) | Strontium Removed Over 1 Year (ppm) | Final Strontium (ppm) |
|---|---|---|---|---|
| 0.5% of tank volume | 8 | 0.5 | 34 | none |
| 1% of tank volume | 8 | 1 | 67 | none |
| 2% of tank volume | 8 | 2 | 134 | none |
From these results, it is obvious that the potential for strontium depletion is significant. Strontium has not, however, been so extensively depleted in my aquarium using this material. Perhaps water changes have made all the difference. Or perhaps there are other processes that impact import or export of strontium. Nevertheless, the potential for strontium depletion seems obvious.
Comparison of limewater to CaCO3/CO2 reactor media
In previous articles, I have used published data to show how magnesium1 and strontium2 might become depleted over time in aquaria using calcium carbonate/carbon dioxide reactors. Table 9 shows the relative concentrations of calcium, magnesium, and strontium in these different systems. From this table, it is clear that limewater is not alone in delivering too little strontium, with the commercial reactor media being similarly deficient. The reactor media are better at delivering magnesium than settled limewater, since the lime starts out about the same as the reactor media, but then looses its magnesium to precipitation.
| Supplement | Mg/Ca | Sr/Ca |
|---|---|---|
| Solid Quicklime | 0.0038 | 0.00037 |
| Settled Limewater | 0.000028 | 0.00039 |
| Koralith CaCO3 | 0.0024 | 0.000065 |
| Super Calc Gold CaCO3 | 0.0070 | 0.000014 |
| Quarried Limestone | 0.010 | 0.00037 |
| Nature’s Ocean crushed coral | 0.0065 | 0.00073 |
| A Typical Coral | 0.025 | 0.02 |
Conclusions
Many aquarists, myself included, have used quicklime from the Mississippi Lime Company to supplement calcium and alkalinity. I have been using it for several years, and know aquarists that have been using it much longer than that. Using such a material, however, has the potential to deplete both magnesium and strontium over time. The effect is especially pronounced for strontium. The depletion mechanism is simple: the dosed limewater simply adds less magnesium and far less strontium than corals in the ocean normally deposit. No unusual precipitation reactions are necessary for the aquarium to become depleted. In fact, the same depletion mechanisms take place for aquarists using CaCO3/CO2 reactors and any of several commercial CaCO3 media.
Whether these ions actually become depleted in aquaria will depend on many factors, such as the use of regular water changes, the import of such ions in other ways, and whether corals and coralline algae (and abiotic precipitation) use up strontium and magnesium to the same extent that they do in the ocean. I am nevertheless surprised that the strontium in my aquarium (about 15 ppm)2 is about the same as in the Instant Ocean salt mix that I use, and does not appear to be significantly depleted.
The results obtained in this study apply only to the particular brand of bulk lime tested. It is not known if any of the commercial aquarium supply companies use this bulk material, or its hydrated form, to make their “kalkwasser” products. The results on settled limewater involving magnesium will likely extrapolate to all brands, as the precipitation of magnesium will be largely independent of how much magnesium is present to begin with. Strontium, however, could vary significantly.
Nevertheless, these results suggest that aquarists using limewater, as well as those using CaCO3/CO2 reactors, should be aware of the fact that both strontium and magnesium may become depleted over time.
Happy Reefing
ICP Method Details
ICP was performed on a Varian ICP-OES (Inductively Coupled Plasma- Optical Emission Spectroscopy) instrument. In order to get actual concentration values, I compared the emissions for each ion at 4 different emission wavelengths. These were:
- Calcium: 317.933, 393.366, 396.847, and 422.673 nm
- Magnesium: 279.553, 279.800, 280.270, and 285.213 nm
- Strontium: 215.283, 216.596, 407.771, and 421.552 nm
Thee different wavelengths have different emission intensities. In some cases, the signal was too strong, and samples were diluted, or that emission wavelength was simply not used for that ion in that sample. ICP-OES is so sensitive to these ions that even the pure (18 MW) laboratory deionized water showed clear emission peaks from these ions at the most sensitive wavelengths.
None of the samples were filtered, but all looked visibly clear.. The quicklime sample was made suspending 1 g of quicklime (Mississippi Lime Co. Vertical Calcium Oxide: CODEX (Food Grade) Pulverized Quicklime; Figure 1) in 1 L of deionized water. The sludge sample was prepared using 2.9 g of sludge (a mixture of water and solids) in 1 L of deionized water. The solid quicklime and sludge samples were acidified to pH 2 using reagent grade concentrated hydrochloric acid to be sure it all dissolved.
Quantitation involved comparison of emission intensity to standards made from calcium sulfate and magnesium sulfate, and a commercial strontium standard (50 ppm) supplied by Varian. Calcium standards were 294, 29.4 and 2.94 ppm calcium. Magnesium standards were 98.6, 9.86, and 0.986 ppm magnesium. Strontium standards were 5, 0.5 and 0.05 ppm strontium. The signal from deionized water acidified with the same amount of acid was subtracted from those samples that required acidification (that is, the solid quicklime and sludge samples). This subtraction turned out to be insignificant for magnesium, but was significant for strontium. ICP-OES is a very sensitive technique for these ions, and a real signal could easily be detected for all three, even in the pure (18 MW) laboratory deionized water.
References
- Magnesium in Reef Aquaria by Randy Holmes-Farley, Advanced Aquarist, October 2003 http://www.advancedaquarist.com/2003/10/chemistry
- Strontium and the Reef Aquarium by Randy Holmes-Farley, Advanced Aquarist, November 2003 http://www.advancedaquarist.com/2003/11/chemistry
- Magnesium Ion Precipitation in Reef Aquaria: A Tempest in a Teapot by Craig Bingman, Aquarium Frontiers, July 1997. http://www.animalnetwork.com/fish2/aqfm/1997/jul/bio/default.asp
- Limits To Limewater, by Craig Bingman Aquarium Frontiers, January/February 1997.
- Limits To Limewater…Revisited by Craig Bingman, Aquarium Frontiers, August 1999. http://www.animalnetwork.com/fish2/aqfm/1999/aug/bio/default.asp
- What is Alkalinity? By Randy Holmes-Farley Advanced Aquarist, February 2002. http://www.advancedaquarist.com/2002/2/chemistry
- The Chemical & Biochemical Mechanisms of Calcification in Corals by Randy Holmes-Farley Advanced Aquarist April 2002. http://www.advancedaquarist.com/2002/4/chemistry
- Solutions to pH problems, by Randy Holmes-Farley http://www.advancedaquarist.com/2002/6/chemistry
- Jaubert’s Method, the “Monaco System,” Defined and Refined, By Julian Sprung http://www.advancedaquarist.com/2002/9/aafeature
- The Relationship Between Alkalinity and pH, by Randy Holmes-Farley http://www.advancedaquarist.com2002/5/chemistry
- It’s in the water, by Ronald Shimek http://reefkeeping.com/issues/2002-02/rs/feature/index.htm
- It is still in the water, by Ronald Shimek http://reefkeeping.com/issues/2002-03/rs/feature/index.htm
- Metals in Limewater by Randy Holmes-Farley, Advanced Aquarist, May 2003 http://www.advancedaquarist.com/2003/5/chemistry
- The Degradation of Limewater in Air by Randy Holmes-Farley Reefkeeping May 2003 http://reefkeeping.com/issues/2003-05/rhf/feature/index.htm




0 Comments