This study was carried out to determine if exposure to copper would cause mortality in flame angelfish (Centropyge lorciulus) and affect reproduction of the orchid dottyback (Pseudochromis fridmani). Flame angelfish were exposed to copper in a series of toxicity experiments. In the first assay, angelfish were exposed to copper at 0.00, 0.05. 0.10, 0.15, 0.20 and 0.25mg/L for a period of 48 hours (n=5). In the second experiment, angelfish were exposed to copper at 0.00, 0.10, 0.15 and 0.20mg/L for 196 hours (n=8). In a third experiment, orchid dottyback broodstock pairs (n=3) were maintained and monitored for reproductive performance (spawn frequency, fecundity, fertilization rate and survival of hatched larvae) while in copper-free water (0.00mg/L) or water treated with copper (0.10mg/L). Results of the toxicity experiments revealed that flame angelfish were accutely sensitive to copper in the first trial, where 60% of the fish exposed to the
0.25mg/L level died within the first 12 hours of exposure. Likewise, flame angelfish exposed to 0.15 and 0.20mg/L exhibited 40% mortality. In the second assay, flame angelfish also exhibted increased mortality (25%) at the highest level tested (0.20mg/L), though the onset of mortality, in that experiment, was delayed until after 120 hours of exposure. Results of the third experiment demonstrated that copper exposure at 0.10mg/L significantly reduced fecundity and negatively affected embryonic development from orchid dottyback broodstock. However, upon replacement into copper-free water, subseqent fecundity, embryonic development and larval survival characteristics were not significantly different from their pre-exposure values. The results of these combined experiments indicated that elevated copper levels can cause accute mortality in flame angelfish and significantly reduce the reproductive performance of orchid dottyback broodstock. Therefore, the use of copper as a therapy for external parasites in these species is cautioned.
Introduction
During the commissioning of a new marine ornamental fish broodstock laboratory at The University of Maine, we experienced an episode of acute mortality of nearly all our flame angelfish broodstock in response to what appeared to be an unidentified toxin in the system. Abruptly one day in June 2004, our flame angelfish were observed to be swimming erratically at the surface of their tanks and some appeared to be “gasping.” Closer examination of all our angelfish revealed that most were exhibiting rapid respiration and some appeared listless. The angelfish were promptly removed from the system and placed into new tanks in another system. Unfortunately, most of the fish succumbed to the apparent toxin prior to being moved. Those fish that had survived the move, recovered rapidly once in new water. Other fish species in the system (anemonefish and dottybacks) appeared normal and otherwise unaffected; however, their spawning performance and egg quality did significantly decline for a prolonged period following this event. After more than two months of investigation, we concluded that the cause of the mortality was likely an elevated copper level in the system caused by the accidental dropping of copper wire into one of the sumps in our recirculation system.
About two weeks prior to the incident, an electrical junction box was installed in an area near our main filtration area. Upon inspection of our systems, we found pieces of copper wire insulation and small clippings of mostly dissolved copper wire in the system sump, near the main pump intake. It is likely that these clippings were byproducts of the recent electrical work, and were accidentally dropped into the system. Months after the copper wire was found and removed, the system continued to exhibit an elevated copper level of 0.10 to 0.20mg/L, which is nearly the therapeutic level for treatment of marine fish parasites (Noga, 2000). Since copper was never utilized as a therapy in this system, it was not suspected as a possible toxin and the actual copper level at the time of the fish mortalities was not recorded. Therefore, it is possible that the copper level at the time of the mortality was considerably higher, as we had made several large (>50%) water changes to
the system immediately following this event, and prior to measuring the dissolved copper levels. What remained unclear is why only the angelfish exhibited this acute response, while the other species in our system appeared mostly unaffected.
Copper is an essential element required by all living organisms, but it can be toxic to aquatic species when present at elevated concentrations (Grossel et al., 2003, 2004a, 2004b; Handy, 2003). For example, copper is widely used as an algaecide and mulluscicide (Paris-Palacios et al., 2000) and frequently employed as a treatment for pathogenic parasites of fish (Bassleer, 1996; Noga, 2000; Perschbacher, 2005). Although toxicity arising from dietary exposure to copper generally only occurs at very high levels, exposure to low levels of dissolved copper in the holding water can cause toxic effects in aquatic organisms (Grossel et al., 2004a).
While the mechanisms of copper toxicity of freshwater fish have been studied in some detail, much less is known about the mechanisms of copper toxicity of marine teleost fish (Grossel et al., 2003). It has been suggested that marine fish are generally less sensitive to water borne copper exposure than freshwater fish (Grossel et al., 2003). Calcium and sodium in the water have been found to reduce both copper uptake and toxicity in freshwater fish and it is suggested that the high concentrations of these ions in seawater help to reduce the acute sensitivity of marine teleosts to copper (Grossel et al., 2003). Increasing water hardness, addition of organic substances, and increasing pH will also reduce the toxicity of copper in fresh water (Handy, 2003).
The physiological mechanism of acute copper toxicity to fish is well known (reviewed in Taylor et al., 1996). Acute copper toxicity in fish is caused by direct target effects on the gill epithelium (Noga, 2000). Acute copper exposure causes gill edema and epithelial lifting (Handy, 2003). Edema of the gill is followed by accumulation of solutes in the epithelial cells and an influx of water into the cells, leading to loss of ionoregulatory control. Efflux of electrolytes from the blood over the gill epithelium then results in cardiovascular failure and death (Handy, 2003).
While the acute effects of copper toxicity are well studied, the effects of chronic copper toxicity are not well documented (Handy, 2003). However, it has been shown that chronic (4 weeks or more) copper exposure can result in altered bodily functions and physiological changes across a range of body systems. The physiological processes that have been found to be affected by chronic copper exposure include altered cell type and turnover in gut epithelium, changes in ionoregulatory physiology, altered immunity, change of swimming speeds to preserve metabolic scope for aerobic metabolism, and altered reproductive strategy (Handy, 2003).
The objectives of the present study were to:
- Determine if exposure to copper would affect the survival of a commonly traded marine ornamental fish, the flame angelfish.
- Determine if exposure to copper would affect the reproductive performance and embryonic development of a cultured marine ornamental fish, the orchid dottyback.
Due to the ubiquitous use of copper as a treatment for external parasites in the marine aquarium industry and the documented acute and chronic effects of copper on fish, coupled with our unexpected episode with the flame angelfish, we considered it necessary to investigate possible impacts such treatments may have. Our goal was to determine if the level of copper that we recorded after the mortality event could have caused the observed mortality and contributed to the decline in reproductive performance of our other pairs of fish.
Methods
Experiments 1 and 2: Effects of Copper Exposure On Survival of the Flame Angelfish (Centropyge lorciulus)
Experiment 1
The first of a series of copper toxicity experiments was a 48hour trial completed in June of 2005, at the Oceanic Institute (OI) in Hawaii. This first copper toxicity trial investigated the effects of copper exposure on flame angelfish (Centropyge lorciulus) at 0.0, 0.05, 0.10, 0.15, 0.20, and 0.25mg/L. Five replicate fish were tested in each treatment except the control, which contained three replicate fish. This experiment was done in 30 identical (75L) tanks (Fig. 1), with one fish stocked per tank. The majority of fish that comprised each treatment group had been at OI for several weeks prior to the start of the trial and were well acclimated to the aquarium conditions. Approximately two new fish per treatment group were added the day prior to the experiment to increase the number of replicates. Each tank had a separate external filter and light aeration was provided via an air stone in each tank. The tanks were exposed to ambient photoperiod (filtered natural
sunlight) and the temperature was maintained between 24-27 °C. The salinity of the water was approximately 32ppt. New seawater was provided to each tank at a rate of ~25ml/minute (50% exchange per day). Copper was dosed directly into the tanks as copper sulfate using a commercially available preparation (Red Sea “Paracure™”) and the total copper level was determined by colorimetric analysis (Bicinchoninate method; Hach #2504025) twice daily at 0900 and 1500 for each tank using a Hach™ DR/890 colorimeter. When necessary, copper was re-dosed to maintain the desired treatment level. Mortality was monitored hourly for the first 12 hours, followed by every 12 hours for the duration of the experiment.

Figure 1. Photograph of the bioassay system at the Oceanic Institute. Each 75L tank was equipped with separate lighting and filtration and could be run as entirely flow-through, re-circ, or a combination of both.
Experiment 2
The second copper toxicity experiment was completed in January of 2006, using the bioassay system (Fig. 1) at OI, but with a modified configuration. The same 75L tanks were utilized, but they were replumbed to allow for continuous exchange of natural seawater (6 tank exchanges/day). Temperature was maintained at 26-27 °C and salinity was maintained at 32ppt. Additionally, new lighting and filtration were added to each tank in the form of an Eclipse™ hood (Marineland Aquarium Products, Inc.). The lighting system was set on a timer to allow for 14 hours light and 10 hours dark. Four treatment levels (0.0, 0.10, 0.15 and 0.20mg/L copper) were tested, with one fish stocked per tank (n=8). For this experiment, all fish were stocked at the same time (24h prior to start of trial) and originated from a local marine fish importer (Hawaiian Sealife Inc, Honolulu, HI).
Copper was added to the incoming seawater as copper sulfate using Dosatron™ (DI 1500) proportional mixing pumps, which were plumbed into the incoming seawater lines (1 dosing unit per treatment). A 100mg/L copper solution was made daily using distilled water and cupric sulfate pentahydrate (Fisher Scientific). This stock solution was dosed into the incoming seawater lines for the respective treatment groups to maintain the desired treatment levels for the duration of the study. This configuration allowed for very tight control over the copper levels tested while maintaining excellent water quality in the aquariums. Total copper levels were tested once daily for each treatment (n=3 samples/treatment) using colorimetric analysis as described for Experiment 1.
Experiment 3: Effects of Copper Exposure on Reproduction and Embryonic Development of the Orchid Dottyback (Pseudochromis fridmani)
Experimental Conditions and Broodstock Husbandry
This experiment was conducted at the Aquaculture Research Center (ARC) at The University of Maine campus in Orono, Maine. Three orchid dottyback (Pseudochromis fridmani) pairs were selected from a collection of spawning broodstock held by a commercial marine ornamental aquaculture company (Sea & Reef Aquaculture, LLC). Each pair had spawned regularly over the preceding year and had produced numerous viable embryos and larvae. Preceding the experiment, each pair was moved to an isolated 75L glass aquarium. The aquaria were bare, except for a few pieces of PVC pipe, which served as surrogate “dens” for the fish. The water temperature was maintained at 27 ± 1 °C, and salinity was kept at 31ppt. The photoperiod was 14h L: 10h D. Each tank was filtered by a submerged airlift sponge filter, which also served as the aquaria’s biofilters. Water quality parameters were checked daily and maintained at the following: pH, 8.1-8.3; NH3, 0.00-0.30mg/L; NO2, 0.00-0.04mg/L. Approximately 25-30% of the tank water was replaced weekly using an artificial seawater mix (Forty Fathoms- Crystal Sea) combined with reverse osmosis filtered water. The new seawater was mixed and aerated for 24 hours prior to water exchanges. The fish were fed four times daily a complex “mixed” diet consisting of commercial aquarium diets, frozen mysid shrimp and fresh seafood. This feeding regime was identical to their prior regimen as commercial broodstock.
Copper Treatment
The pairs were held in their respective aquaria at 0.00mg/L Cu for 30 days prior to copper treatment (period 1). Copper (as copper sulfate) was administered, as a commercially available preparation (Red Sea “Paracure™”) to each aquarium at 0.10mg/L for a period of 21 days (period 2). Copper levels were tested twice daily on replicate (n=3) 10ml samples of aquarium water. Copper measurements were recorded by colorimetric analysis using a Lamotte “Smart Colorimeter™” utilizing the Diethldithiocarbamate method (Lamotte #3646-SC). Copper levels were maintained at 0.10 ± 0.02 mg/L through twice daily doses of copper sulfate. Following the 21-day exposure period, each pair was held for an additional 30 days in copper-free water (period 3).
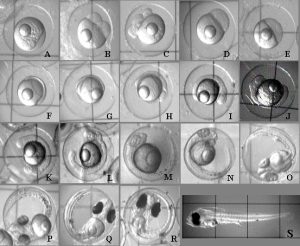
Figure 2. Photomicrograph series depicting the normal embryonic development of the orchid dottyback (Pseudochromis fridmani). Time = time post fertilization. A 20min; B 45min; C 90min; D 4h; E 8h; F 11h; G 14h; H 17h; I 19h; J 21h; K 24h; L 29h; M 34h; N 39h; O 45h; P 64h; Q 72h; R 91h; S 93h. Box grid on slide = 1mm.
Sampling Procedures
Spawn quality information consisting of total number of eggs produced, percent normal embryos at 24h post-fertilization, percent normal embryos at 72h post-fertilization, percent hatch, and total number of larvae to survive 12h post-hatch was collected from each pair over the duration of the experiment. Three to four spawning cycles were recorded for each pair during each of the three treatment periods. Each pair was monitored daily for the production of eggs. Time of spawning was recorded, and eggs were removed from the tank for initial analysis at approximately 24 hours post-fertilization.
The entire egg mass was removed from each aquarium and carefully blotted dry with lint-free paper. The egg mass was then transferred into a 25ml graduated cylinder containing a known volume of aquarium seawater. The amount of water displaced by the egg mass was recorded to the closest 0.10ml. Knowing the average volume per egg, this displacement method (Shirley, unpub. data) was used to calculate the approximate number of eggs produced per spawn. The egg mass was replaced after a small sub-sample of the egg mass (50-100 eggs) was removed at 24 and 72 hours post-fertilization and analyzed under a dissecting microscope.
The embryos were observed for any developmental abnormalities as compared to a “normal” developmental series (Fig. 2). The number of normal and abnormal embryos at each sampling session was recorded. On the evening of expected hatch (90 hours post-fertilization), the egg mass was removed from the aquarium to hatch in an 8 liter aquarium (Fig. 3). The egg mass was placed in a submerged 250ml Erlenmeyer flask and gently aerated with a rigid airline tube producing approximately 40 bubbles per minute. This motion facilitated hatch of the egg mass once the lights went out. At approximately 12 hours post-hatch, the surviving hatched larvae were euthanized with MS-222 (100mg/L) and counted.
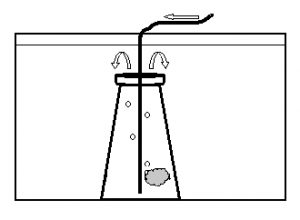
Figure 3. Schematic diagram of the aquarium used to hatch dottyback eggs. Arrows indicate direction of air/water flow. The egg mass was aerated in a 250 ml flask, submerged in an aquarium. Top of the flask was open to allow hatched larvae to swim out.
Statistical Analysis
All data were analyzed using SYSTAT™ (ver 11.0). Data from Experiment 3 were analyzed using a one-way ANOVA with repeated measures. Normality of the data (Shapiro and Wilk, 1965) and homogeneity of the variance (Snedecor and Cochran, 1993) were tested to ensure assumptions for ANOVA were satisfied. Percent data were transformed (arcsine) before conducting analysis of variance. Tukey’s HSD test (Snedecor and Cochran, 1993) was used to determine significant differences among the means (p<0.05).
Results
Experiment 1
The daily mean concentration of copper measured for each treatment and the respective survival to 48 hours are presented in Table 1. Targeted copper concentrations were rarely achieved and were difficult to maintain at the desired treatment levels for more than 8 hours. Generally, recorded copper levels were below the targeted values in the morning and above targeted values in the afternoon following re-dosing. Additionally, fluctuating background levels of copper in our incoming seawater were recorded between 0.01-0.04mg/L, which further complicated proper dosing. Despite the fluctuations in copper levels, angelfish were acutely sensitive to copper at the higher doses. Flame angelfish in treatments that received the two highest levels of copper exhibited significant mortality within the first 12 hours of exposure.
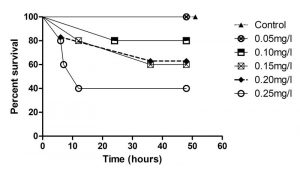
Figure 4. Survival of flame angelfish exposed to six levels of copper for 48 hours (Control n=3; all others n=5).
| Mean Copper Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Day 1 | Day 1 | Day 2 | Day 2 | Day 3 | Treatment Mean | STDEV | % Survival | |
| mg Cu/L | AM | PM | AM | PM | AM | for Duration | 48 hrs | ||
| 1 n=5 individuals for all copper exposed fish, n=3 individuals for control (0.00mg/L) treatment | |||||||||
| 0.00 | 0.01 | 0.04 | 0.00 | 0.04 | 0.00 | 0.02 | 0.02 | 100% | |
| 0.05 | 0.04 | 0.09 | 0.01 | 0.10 | 0.02 | 0.05 | 0.04 | 100% | |
| 0.10 | 0.04 | 0.14 | 0.06 | 0.15 | 0.04 | 0.08 | 0.06 | 80% | |
| 0.15 | 0.06 | 0.18 | 0.11 | 0.17 | 0.08 | 0.12 | 0.05 | 60% | |
| 0.20 | 0.13 | 0.22 | 0.12 | 0.22 | 0.05 | 0.15 | 0.07 | 60% | |
| 0.25 | 0.18 | 0.26 | 0.12 | 0.26 | 0.16 | 0.20 | 0.06 | 40% | |
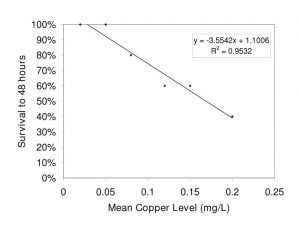
Figure 5. Relationship of flame angelfish survival to increased copper level after 48 hours of exposure. (0.00mg/L n=3; all others n=5)
The relationships between exposure duration and flame angelfish mortality at increasing copper concentration are illustrated in Figure 4. At 6 hours of exposure, the 0.25mg/L treatment had already experienced 40% mortality. At 12 hours of exposure, the 0.25mg/L treatment experienced a total of 60% mortality, whereas the 0.20mg/L and 0.15mg/L treatments experienced 20% mortality. After 24 hours, the 0.1, 0.15 and 0.20mg/L treatments experienced an additional 20% mortality. After 36 hours of exposure, the cumulative mortality had reached its maximum point and the values were constant until the conclusion of the experiment at 48 hours. All of the fish in the 0.00mg/L (n=3) and 0.05mg/L (n=5) treatments survived the trial. Results of a log-rank (Mantel-Cox) comparison of the survival curves revealed a significant difference (p<0.05) between the slopes of the 0.25mg/L and Control treatment curves. These data indicated that copper toxicity increased with increasing concentration and that mortality was strongly correlated with copper level. Figure 5 illustrates the relationship between the copper levels recorded and mortality in flame angelfish at the conclusion of this experiment.
Experiment 2
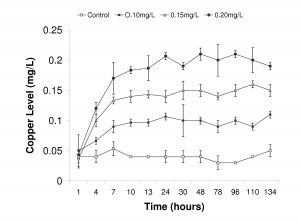
Figure 6. Recorded copper levels during trial Experiment 2. Copper levels were measured once each day on three replicate samples and values are reported as means ± standard deviation.
Copper levels were maintained at controlled levels in Experiment 2 and did not fluctuate significantly from the targeted treatment levels (Fig. 6). However, due to the use of the Dosatron™ on the flow-through seawater configuration, copper levels did not reach targeted concentrations until after 24 hours. We routinely measured values between 0.02-0.05mg/L of copper in the control treatment, indicating a background level of copper in our incoming source water. These background values were similar to what was observed in Experiment 1 (Table 1).
The survival of flame angelfish exposed to the 4 levels of copper is shown in Figure 7. In contrast to what was observed in Experiment 1, mortality was not observed until after 5 days of exposure at the highest level (0.20mg/L). After 144h of exposure (day 6), the 0.20mg/L treatment had experienced 25% mortality. No additional mortality was observed for the duration of the experiment. None of the other treatment groups experienced any mortality.
Experiment 3
A summary of the data collected during experiment 3 is presented in Table 2. During the 30-day pretreatment period, each pair of dottybacks spawned between four and five times. The mean number of eggs produced per spawn (n=14) was 1927 ± 122 and the mean number of hatched larvae counted at 12 hours post hatch was 730 ± 135. Normality of the developing embryos was 98% and 84% at 24h and 72h, respectively. Embryonic development closely followed the normal progression as depicted in Figure 2. The mean hatch rate was 46%. It is important to note that we believe the hatch rate was negatively affected by the artificial incubation of the egg mass, as typical hatch rates are normally near 100% when the egg mass was left under the male’s care. Despite our best efforts to replicate the motion and hatching environment of the egg mass, we typically could not achieve higher than 70% hatch rates.
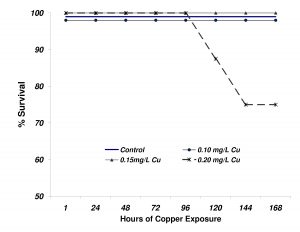
Figure 7. Survival of flame angelfish exposed to copper for 168 hours. Treatment levels were not reached until after 24 hours of dosing (n=8 fish per treatment).
| Period | # Spawns | Eggs/Spawn | Normal 24 hrs % | Normal 72 hrs % | Hatched Larvae | Hatch Rate % | |
|---|---|---|---|---|---|---|---|
| Values are means ± standard error. | |||||||
| Pair 1 | Pre-Cu | 4 | 2365 ± 203 | 97.37 ± 1 | 96.90 ± 2 | 1107 ± 46 | 54.07 ± 6 |
| Pair 2 | Pre-Cu | 5 | 2013 ± 96 | 98.72 ± 1 | 93.89 ± 2 | 941 ± 91 | 49.12 ± 8 |
| Pair 3 | Pre-Cu | 5 | 1491 ± 122 | 99.13 ± 1 | 60.97 ±23 | 376 ± 193 | 33.59 ± 19 |
| Pair 1 | During Cu | 4 | 1305 ± 66 | 56.73 ± 9 | 0 | 0 | 0 |
| Pair 2 | During Cu | 3 | 963 ± 296 | 49.26 ± 10 | 0 | 0 | 0 |
| Pair 3 | During Cu | 2 | 746 ± 186 | 37.78 ± 7 | 0 | 0 | 0 |
| Pair 1 | Post-Cu | 3 | 2641 ± 55 | 95.30 ± 2 | 91.58 ± 3 | 1734±433 | 69.76 ±18 |
| Pair 2 | Post-Cu | 4 | 1608 ±70 | 72.80 ±18 | 53.36 ± 22 | 497 ±316 | 32.29 ±20 |
| Pair 3 | Post-Cu | 0 | N/A | N/A | N/A | N/A | N/A |

Figure 8. (Top) Mean number of eggs and hatched larvae produced and (Bottom) mean percent normal embryos at 24 and 72 hrs post fertilization, before, during and after copper treatment. Values are reported as means (n=3 pairs) ± standard error. Asterisk indicates significant differences between means (p<0.05).
During the 21-day copper treatment period (0.10mg/L Cu), the three pairs of dottybacks spawned 2, 3 and 4 times respectively. The mean number of eggs produced per spawn (n=9) was 1004 ± 162, which was significantly fewer (p<0.05) than during both the pre-treatment and post treatment periods (Fig. 8A). Normality of the developing embryos (Fig. 8B) was also significantly lower at 24h (47%) and at 72h (0%) than during pre and post-treatment periods. The mean number of hatched larvae produced was 0, as all of the developing embryos had died by 72 hours post-fertilization.
Most of the abnormalities caused by the copper treatment were visible by 24h post-fertilization (Fig. 9). Exposure to copper at the levels tested caused many of the developing embryos to arrest shortly after fertilization. These arrested embryos would start to darken in color as they decomposed within the egg. Many of the eggs appeared less round, and in some cases were completely misshapen.
Despite the large number of abnormalities observed at the 24 hours post-fertilization sampling period, many of the embryos continued to develop until about 30 to 40 hours post-fertilization. At the 72 hours post-fertilization sampling period it was clear that almost none (<1%) of the embryos exposed to copper sulfate had survived (Fig. 10).
Due to the fact that we allowed the male to care for the egg mass until just prior to hatch, very few eggs were available for sampling at the 72h sampling period during the copper treatment. It is common for the male to cull out dead or improperly developing eggs during the incubation period, thereby reducing the number of embryos we were able to evaluate. However, of the eggs remaining for sampling, we were still able to document abnormal development of embryos exposed to copper (Fig. 10). It appeared that most development had ceased by 40 hours post-fertilization, despite normal development until that point. There were a very small (<1%) number of embryos that completed normal development to 72 hours post-fertilization. However, the male had consumed, or otherwise culled out, all remaining eggs from his den prior to the 90hr post-fertilization point when we would normally remove the egg mass for hatching. Therefore, there were no eggs available for hatching, resulting in zero hatched larvae during the copper treatment period.

Figure 9. Photographs of abnormalities in early embryonic development of orchid dottyback eggs exposed to copper. Arrows indicate normal embryos. A) Normal 24hr post-fertilization embryo surrounded by dead (dark) and misshapen eggs. B) Close-up of arrested 24hr post-fertilization embryo, showing characteristic darkening of yolk area. C) Normal 24hr post fertilization embryo with embryo that arrested 10-12hrs post-fertilization. D) Normal 24hr post-fertilization embryo surrounded by embryos that arrested 4-8hrs post-fertilization.
During the 30-day recovery period (period 3), pair #1 and #2 spawned three and four times, respectively. The mean number of eggs produced per spawn (n=7) was 2050 ± 204 and the mean number of hatched larvae counted at 12 hours post-hatch was 1027 ± 318. Normality of the developing embryos was 82% and 69% at 24h and 72h, respectively. Embryonic development closely followed the normal progression as depicted in Figure 2. The mean hatch rate was 48%. All spawning data collected in the post-treatment period were not significantly different from the pre-treatment period. However, pair 3 did not resume spawning during the 30-day post-treatment observation period. It is unknown why they did not resume spawning, as all other observed behaviors appeared normal.
Discussion
In the summer of 2004, acute mortality of flame angelfish broodstock and a gradual, but system-wide decline in reproductive performance (egg output and egg viability) across a range of other species (clownfish & dottybacks) was observed. After much investigation, it was determined that the only abnormal system parameter was an elevated copper level, caused by the introduction of copper wire into the sump of the recirculating holding system. The wire had slowly dissolved releasing copper into the water and eventually elevated the system level to 0.10-0.20mg/L. It was at least 6 weeks before the elevated copper level was discovered.
After much effort, the copper was removed from the system using a series of large water changes, removing all calcium carbonate substrate (which absorbed copper, acting as a copper sponge, subsequently re-releasing copper back into the water to equilibrium), and by using a commercially available copper removing resin Cuprisorb™ (Seachem Laboratories, Inc.). Once the copper level was reduced to below 0.10 mg/L, most of the broodstock pairs began to spawn normally again.
Figure 10. Photographs of abnormalities in late embryonic development of orchid dottyback eggs exposed to copper. Arrows indicate normal embryos. A) Close-up of arrested ~35hr post-fertilization embryo, showing darkening of yolk area and bent body axis. B) Normal 72hr post-fertilization embryo next to an embryo that arrested 30-40hrs post-fertilization.
The use of copper as a treatment for marine parasites requires constant exposure to levels between 0.15-0.25mg/L for a minimum of 21 days (Bassleer, 1996; Noga, 2000). Given the demonstrated sensitivity of angelfish to copper, copper treatment is counter indicated for this species. In strong contrast to the low-level treatments, fish in the highest-level treatments (Experiment 1) were immediately adversely affected by the addition of copper to the water. Within a few hours of exposure, many fish in the high-level treatments were exhibiting signs of stress (rapid respiration and erratic swimming behavior). The behaviors observed in these treatments were very similar to those observed during the acute mortality event at the ARC in Maine. It was clear that the addition of copper to the water at recommended therapeutic levels caused severe stress and injury to these fish.
Prior to the start of Experiment 1, mortality of two of the recently acquired fish was recorded. Those two fish had been observed showing signs of “stress” (rapid breathing and abnormal swimming behavior) upon arrival at OI. They were purposefully going to be assigned to the 0.00mg/L treatment group with the idea that any additional treatment “stress” might inadvertently cause mortality, confounding the effects of the copper treatment. However, mortality of these fish prior to the experiment caused the trial to commence with only 3 replicates in the control treatment.
This observed mortality could be attributed to the stress imposed on fish when they are recently imported. The only control treatment fish that were lost were the new fish, acquired just prior to the experiment. Acclimation stress, and subsequent mortality, is unfortunately common in newly acquired fish. Mortality is particularly common with fish that are recently collected, as these undoubtedly were (coming directly from an importer). The remaining fish in the control treatment displayed no visible signs of stress and appeared completely normal for the duration of the trial. Also, the other newly acquired fish did not contribute disproportionately to the observed mortality in the other treatment groups. Furthermore, angelfish in the 0.05mg/L treatment did not experience any mortality, indicating that this species should not be negatively affected at the background levels of copper routinely measured in the incoming saltwater (derived from wells) at the Oceanic Institute.
In Experiment 2, the same acute onset of mortality following copper exposure was not recorded. However, flame angelfish at the highest treatment level did experience 25% mortality. It is possible that through the more gradual and constant addition of copper, these fish were able to somewhat acclimate to higher copper levels. More likely, as these fish are increasingly sensitive to copper at the higher treatment levels, the reduced fluctuation in copper dosing precluded exposure to these “upper maximums”. In Experiment 1, the targeted treatment levels were often exceeded in the afternoon, exposing the fish (even if only for an hour or two) to higher than desired levels. This exposure could likely have led to increased stress and reduced capacity for coping with the copper at lower levels and/or the ability to adjust to copper fluctuations. However, the former method of dosing copper (not using dosing equipment) is the only feasible method for hobbyists.
Prolonged exposure to copper, with reduced mortality, may be possible by utilizing a flow-through system with dosing equipment, such as the one described in Experiment 2. In this way, precise copper dosing at targeted therapeutic levels may be achieved. However, implementation of such systems is likely beyond the capacity of most hobbyists, importers or retail proprietors. Additionally, it is unknown whether prolonged exposure beyond the one-week end point of this trial would have lead to additional chronic mortality. It is also possible that other, less obvious, physiological injury could be caused by repeated or prolonged exposure to copper. Therefore, alternate treatment strategies should be investigated for the treatment of external parasites in this species of fish.
Results from Experiment 3 verify that the lowest level of copper observed during the period of accidental copper introduction (0.10mg/L) was high enough to impair the reproduction of marine ornamental fish broodstock. The effects of copper on reproduction in marine ornamental fish appear to be two-fold, negatively affecting both the adults and embryos. First, copper at 0.10mg/L affected the adult female orchid dottyback as evidenced by significantly reduced egg production. Second, the same copper level was toxic to the developing dottyback embryos, as none of the embryos survived past 48 hours of exposure. It is interesting to note that although the copper affected the adult females, the adult males did not seem to be affected to the same degree, as evidenced by unchanged fertilization rates of the eggs during copper exposure. Fertilization rates remained near 100% for the duration of this study and did not appear to be affected by copper levels in the water. It was
observed that eggs produced in copper-treated water, if moved to copper-free water shortly after fertilization, would complete normal embryonic development. This result is highly suggestive that the negative effects of copper on the developing embryo occurred after fertilization and were caused by exposure to copper in the water, rather than exposure inside the female during egg maturation.
Although copper exposure impaired the reproduction and embryonic development in this species, those effects were reversed shortly after returning the fish to copper-free water. Following the treatment, two of the three pairs immediately began producing eggs and larvae in similar numbers to pre-treatment levels. Although the third pair did not resume spawning during the 30-day, post-exposure evaluation period, that pair did begin spawning again shortly thereafter. These results indicate that moderate exposure to copper may not have negative long-lasting effects on reproduction in marine ornamental fish. However, caution must be utilized during therapeutic use of copper, as some species, such as flame angelfish, appear to be much more sensitive than others. From these results, it can concluded that the recorded copper levels observed in the system at the ARC could have contributed to the flame angelfish mortality and prolonged reproductive decline in the other marine ornamental broodstock. Copper also proved very difficult to remove from recirculating systems and therefore should be avoided if other viable treatment alternatives are available.
Acknowledgments
Thank you to Søren Hansen, for assistance in identifying and correcting the copper contamination of our broodstock system. We wish to thank Dr. Michael Optiz for preparation of tissue samples and assistance in diagnosis of copper toxicity. Additional provisions for replacement broodstock and systems modifications were provided by The University of Maine. We also wish to thank Kenneth Liu, and Joe Aipa for assistance during copper toxicity assays at the Oceanic Institute. Funding for the angelfish toxicity assays were provided through the Hawaii Sustainable Fisheries Development Project (NOAA Award No. NA05NM4441228).
References
- Ali, A., Al-Ogaily, S.M., Al-Asgah, N.A., Gropp, J., 2003. Effects of sublethal concentrations of copper on the growth performance of Oreochromis niloticus. Journal of Applied Ichthyology 19, 183-188.
- Bassleer, G., 1996. Diseases in Marine Aquarium Fish: causes, symptoms treatment. Basleer Biofish, Westmeerbeek, Belgium. 94p.
- Grossel, M., Hansen, H.J.M., Rosenkilde, P., 1998. Cu uptake, metabolosim and elimination in fed and starved European eels (Anguilla Anguilla) during adaptation to water-borne Cu exposure. Comparative Biochemistry and Physiology Part C. 120, 295-305.
- Grossel, M., McDonald, M.D., Walsh, P.J., Wood, C.M., 2004. Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta) II: copper accumulation, drinking rate and Na+/K+ – ATPase activity in osmoregulatory tissues. Aquatic Toxicology 68, 263-275.
- Grossel, M., McDonald, M.D., Wood, C.M., Walsh, P.J. 2004. Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta) I. Hydromineral balance and plasma nitrogenous waste products. Aquatic Toxicology 68, 249-262.
- Grossel, M., Wood, C.M., Walsh, P.J., 2003. Copper homeostasis and toxicity in the elasmobranch Raja erinacea and the teleost Myoxocephalus octodecemspinosus during exposure to elevated water-borne copper. Comparative Biochemistry and Physiology Part C. 135, 179-190.
- Handy, R., 2003. Chronic effects of copper exposure versus endocrine toxicity: two sides of the same toxicological process? Comparative Biochemistry and Physiology Part A. 135, 25-38.
- Noga, E.J., 2000. Fish Disease: Diagnosis and Treatment. Blackwell Publishing.Ames, Iowa. 367p.
- Paris-Palacios, S., Biagianti-Risbourg, G., Vernet, G., 2000. Biochemical and (ultra) structural hepatic perturbations of Brachydanio rerio (Teleostei, Cyprinidae) exposed to two sublethal concentrations of copper sulfate. Aquatic Toxicology 50, 109-124.
- Perschbacher, P.W., 2005. Temperature effects on acute copper toxicity to juvenile channel catfish Ictalurus punctatus. Aquaculture 243, 225-228.
- Shapiro, S. S., Wilk, M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika 52, 591-611.
- Snedecor, G. W., Cochran, W. G., 1993. Levene’s test of homogeneity of variance. In: Statistical Methods, 8th edition. Iowa State University Press, Ames Iowa.



0 Comments