
First, a little history… There had been a lot of activity, experimentation, and commentary in the late 60s and early 70s on the possibility of rearing marine tropical fish in numbers but no one had yet succeeded. I was lucky; I was in the right place at the right time with the right experience. I had worked from 1969 to 1971 developing the technology for controlled spawning and larvae culture of the Florida pompano, and from that I knew what marine fish larvae needed in order to survive. I also picked the right fish with which to begin the work. If I had picked, Oh, pigmy angelfish, for example, I might be still working at it. I explained in the article why I chose the “Percula” clownfish for the first experiments.
At that point, the hobby did not really distinguish between A. percula, the orange and/or percula clownfish, and A. ocellaris, the common and/or false clownfish. In fact, few hobbyists were familiar with taxonomic conventions and the use of scientific names was only something that those fancy pants scientist types used once in a while. In the hobby, the only clownfish that was typically available was the common clownfish, A. ocellaris. But in the popular literature the percula clownfish was most commonly illustrated, and so the common clownfish that filled the tanks of this fledgling hobby was almost universally given the erroneous common name “Percula clown””. The two species are very close in morphology; there are some subtle color differences and typically 10 dorsal spines in A. percula and 11 dorsal spines in A. ocellaris. A. percula was described in 1802 by Lacepede, and A. ocellaris in 1830 by Cuvier. Given that A. percula was the first described, that name was most used in the early literature, and thus picked up by the early marine aquarists. Books by Gerald Allen and Daphne Fautin eventually ended the lay confusion (almost) between these species. Now, of course, we also have to contend with numerous “breeds” and hybrids of these same species. It is an exciting time for clownfish breeders.
At that time there were relatively few species of marine tropical fish available to hobbyists. Clownfish were among the most popular marine fish, but also quite difficult because most were collected with cyanide. They were also subject to external parasites, and shipment and early captivity mortality was very high. But even with my experience with pompano, it was a culture project with very little extant information to guide the effort; and with limited resources, a lot of experimentation and intuitive guessing on how to resolve problems was required. The thing that always helped me with development of culture procedures with new species was to learn as much as I could about the natural history of the species under culture, the environment, the diet, the reproductive modes, etc., and use that knowledge as a base for development of adequate substitutes for that organism’s basic requirements for survival.
As with the early development of any new technology that holds a promise of commercial value, the early days of marine fish culture were shrouded in secrecy, or least that attempt was made. Thus my article was long on biology and short on technology. It was important, however, to provide enough detail in text and photos to establish with certainty that repetitive culture of relatively large numbers of juveniles had been accomplished. Within a few years, of course, the basic culture techniques, and many improvements as well, soon became relatively well known. Over the years, subsequent articles, books, and websites have provided great detail on the original and many additional techniques. The Marine Aquarium Handbook (now in the 3rd edition) and my book on rearing the orchid dottyback, many very significant recent articles and scientific papers, and books by Hoff, Wilkerson, and Wittenritch have now greatly expanded knowledge and “how to” information on marine ornamental fish culture. And now the information for large and small scale culture of many species (but not enough) of marine fish is readily available. My original article is of historical interest, but still provides good information on the biology of clownfish culture.
Salt Water Aquarium, The international magazine for marine aquarists
Introductory comments from Robert P. L. Straughan, editor, Salt Water Aquarium magazine
March-April, 1973, Volume 9, Number 2
TANK RAISED CLOWNFISH!
SPECIAL SPAWNING ISSUE
A MILESTONE IN THE HOBBY
BREEDING THE CLOWNFISH, Amphiprion ocellaris
By MARTIN A. MOE, JR., MARINE BIOLOGIST
Breeding marine fish has often been termed impossible, improbable, difficult and prohibitively expensive. It is actually none of these, as this article will show; however, it is time consuming and does require a good deal of specialized knowledge. This is not a “how to” article or a scientific paper since the techniques of rearing are still under development and many problems are yet to be solved, but I hope that it will stimulate increased interest in the hobby of maintaining marine fish and show that the prospect of rearing some of the marine tropical fish in large numbers may not be far off.
Fishes of the genus Amphiprion, particularly the common clownfish A. ocellaris (percula) are among the most popular of marine aquarium fish. Their hardiness, vivid coloration, engaging personality and relative abundance are good reasons for their popularity. These traits alone are cause enough to investigate the possibilities of controlled reproduction and a study of what is known of their life history confirms their candidacy for breeding experimentation. Clownfish are demersal spawners, attaching their eggs to hard surfaces near the base of an anemone. They guard and aerate the eggs from spawning to hatching, a period of 7 to 9 days, after the fashion of cichlids. The larvae are large at hatching, about 4 mm in length, have eyes and mouth parts fully formed, and are ready to begin feeding within the first 24 hours of hatching. The adults mature at small size, 50 to 100 mm, and have a limited range, usually the immediate vicinity of an anemone. These biological characteristics make it relatively easy to provide the necessary environment to stimulate natural spawning.
Dr. Gerald R. Allen (1) has compiled a recent and thorough review of the taxonomy and biology of the genus Amphiprion and he reports in this work of rearing 3 A. chrysopterus and one A. tricinctus through the larval stages on dried particulate food, This and other accounts of rearings of Amphiprion (2) (3) and (4) reported in the literature, made A. ocellaris an obvious choice for the first rearing attempts. The following work was conducted partly as an extension of a hobby and partly as independent research on reproduction in marine fishes while attending the University of South Florida.
Spawning

Male and female clownfish above nest during spawning. The male is “mouthing” the eggs and the female is depositing additional eggs on the rock.
The project began in July, 1972, with the construction of two 55 gallon tanks destined to serve as spawning aquaria. Costs were cut markedly through constructing all parts of the tanks and filters with inexpensive, readily available materials. (Perhaps the details of construction will form a later article.) One of these tanks was established with natural sea water and small local fish were used to provide the nitrogenous waste to activate the filter bed. The other was filled with artificial sea water and the filter bed was activated chemically with ammonium chloride. The latter method resulted in a more trouble free tank than the former. Each tank was provided with full spectrum lighting and an attractive decor of construction stone. The photoperiod and temperature were adjusted to provide maximum stimulation of the fish’s endocrine system and then the trauma of establishing the filters began. The tanks were ready for occupation about mid-September. Four A. ocellaris that seemed to be already mated and two large anemones of the genus Stoichactis were purchased at Scott’s Highway Aquarium in St. Petersburg and the experiment began. A diet specially compounded of shrimp, clams, chicken gizzards and certain marine algae and vitamins was fed twice a day. The fish took immediately to the anemones and their general behavior was soon much like that described in the literature for the genus Amphiprion in the wild. The fish spent most of their time bathing in the anemone and hovering just above it protecting their anemone from all real and apparently many imaginary intruders. Movements many feet away from the aquaria would send them charging against the glass or scurrying in rapid retreat into the protective custody of the anemone.
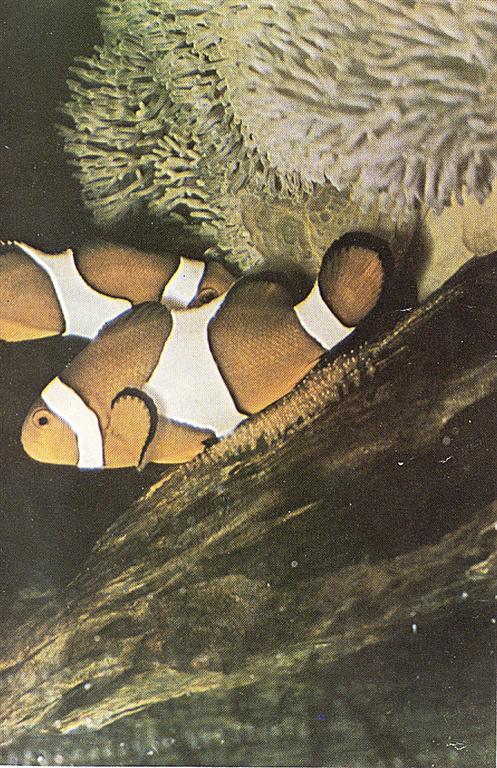
Page 4 color spawn photo–Female clownfish (A. ocellaris)making a pass over the patch of eggs and depositing additional eggs. Male in background is “mouthing” the eggs. Photo by Martin A. Moe, Jr.
Only one pair engaged in spawning activity. This was the pair that occupied the tank established with artificial salt water, although by this time the initial water had been diluted with natural sea water to reduce the nitrate accumulation. The pair in the tank established with natural sea water and local fish had problems with external parasites and had to be treated several times. Also, as they gained in size the suspicion that they were both females became more of a certainty. They are almost equal in size and both have the roundness of the abdomen that seems characteristic of females.
The spawning pair are unequal in size and vary in general shape. The female is the largest of the pair, approximately 80 millimeters long and has a well-rounded abdomen. The male is about 60 millimeters long and has a narrow abdomen and a much smaller appetite than the female. The first spawning occurred on November 3, 1972. There was some indication of impending spawning in the fullness of the female and for a few days before spawning each fish would occasionally bite gently at the abdomen and vent of the other. Spawning occurred, as it did in all subsequent 5 spawns, in the afternoon hours. About one hour before spawning, the pair actively cleans the rock that will receive the eggs by biting the substrate and aggressively jerking the head from side to side. The afternoon spawning could be accurately predicted in the early morning by the extended ovipositor of the female. The ovipositor, a blunt, rather large pinkish organ, did not retract until several hours after spawning. The male’s genital papilla was small, white in color, sharply pointed and appeared only shortly before, during and shortly after spawning. Rapid chasing before spawning or rapid vertical movements as reported by other authors did not occur.
Toward the .end of the surface cleaning activity, the female began to make frequent passes over the rock with the tip of her ovipositor dragging over the cleaned surface. These passes became more frequent until after about 15 minutes the first eggs appeared. These were a bright orange-yellow, about 1 mm in diameter and 2 mm in length. They passed from the ovipositor and immediately adhered by one end to the rock. The female swam in a circular course around and through the cleaned area depositing the eggs as she passed. After every few passes the male swam over the enlarging patch of eggs and fertilized them. No milt (sperm) could be observed in the water, but even eggs placed one or two inches from the main patch were fertilized. During this activity both fish nipped at the anemone and caused it to retract from the area where the eggs were deposited. The nest was always made in an area covered by the anemone.
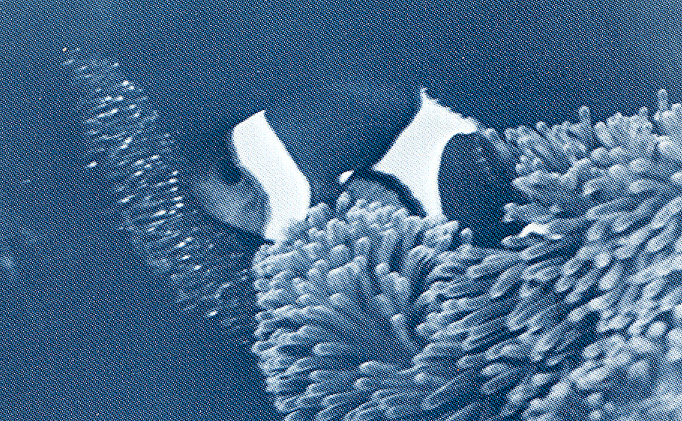
Male guarding the late stage eggs. These eggs are about seven days after spawning and the bright spots of light in the patch of eggs is the flash reflecting from the eyes of the embryos.
The eggs adhered to the rock by means of a short pedestal composed of innumerable small fibers that fanned out over the rock. The yolk contained an oil droplet that may aid the buoyancy of the eggs but did not give them positive buoyancy since they sink if detached from the rock. Each egg could move almost 180 degrees in any direction with water movement, thus the patch of eggs was in constant motion under the careful fanning of the male. The male was clearly in charge of the eggs. He rarely left them for more than a few seconds during the entire period of incubation. The female would give them an occasional mouthing or a quick tail sweep, but did not guard them constantly even though she rarely left the vicinity of the anemone. The male usually positioned himself on topof the eggs and kept them moving with frequent sweeps of his pectoral and caudal fins. He would frequently “mouth” the eggs also, which consisted of positioning himself vertically over the eggs and while maintaining this orientation with quick movements of his fins, he gently bit at them in much the same way as the rock was initially cleaned for spawning. The female also engaged in mouthing the eggs but at more infrequent intervals than the male. This activity continued until the eggs hatched. Although other authors report increased fanning activity the day before the eggs hatch, I did not notice an increase in nest care activities at that time. However, during hatching, which always occurred at night at least several hours after the onset of darkness, the male was constantly active at the nest. This pair spawned 6 times in about 2 months, Nov. 3, Nov. 14, Nov. 30, Dec. 11, Dec. 24, and Jan. 6 were the dates of spawning. Behavior was similar during all spawns and number of eggs spawned varied from an estimated 500 to 800.
Hatching and Larval Recovery

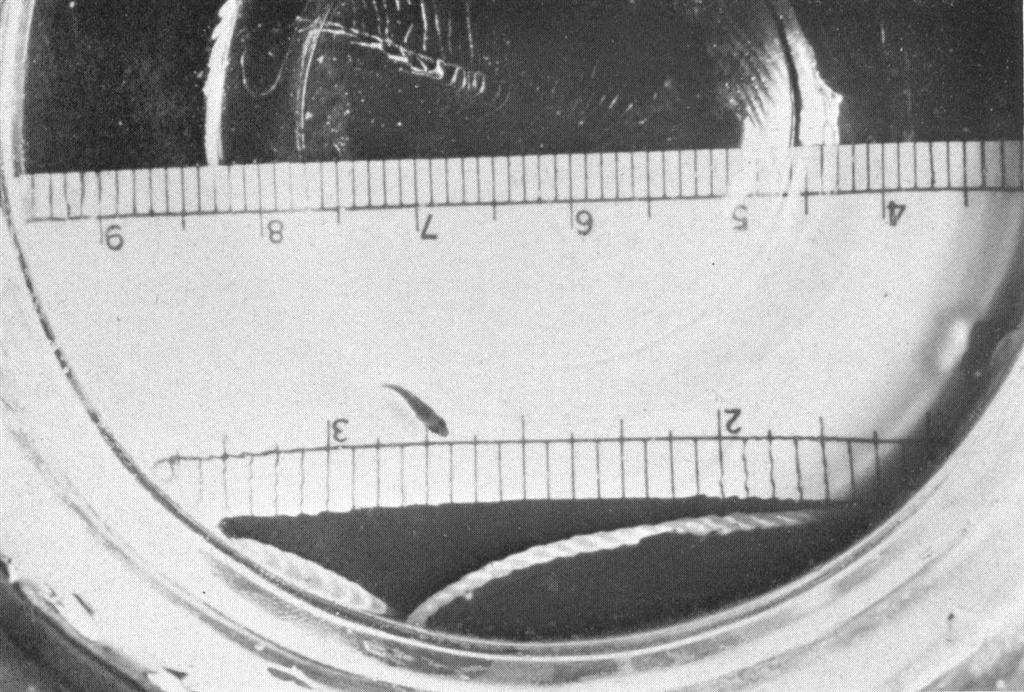
Eggs of the clownfish, Amphiprion ocellaris, about 24 hours before hatching. The eyes and mouth parts are well developed at this stage, but the yolk is still large. The millimeter scale at the top shows the eggs as about 2 mm long and about 2 mm in diameter.
The problem that caused the greatest limiting factor to the number of fish that were reared was not larval mortality, but recovery of the larvae after hatching. This is primarily a manipulative problem and can be solved through experience. Reports in the literature stated that hatching for most Amphiprion takes place on the night of the seventh day after hatching, and sure enough the eggs from the first batch of eggs looked ready to hatch on the seventh day. The reflective pigment of the eyes was well developed, the mouth parts were formed and the embryo was twitching within the chorion (egg shell) of the egg. Recovery of the larvae after hatching in the breeding aquarium was sure to be a struggle and not a positive method of recovery, so that about 75% of the eggs were carefully removed from the rock without disturbing the anemone and with minimal disturbance to the adults. The male resumed care of the remaining eggs when the operation was complete. These eggs, destined to hatch in a few hours (1 thought) were carefully incubated in the prepared larval tanks and I waited anxiously for hatching. It has been said that it’s not nice to fool Mother Nature and I was ready to believe it. The eggs did not hatch in the incubating dish or on the rock. Although egg incubation in the hanging dishes was feasible for a few hours, it was not successful for a period of 30 to 48 hours. The eggs did not hatch until the night of the ninth day and by that time I had taken most of the remaining eggs trying to figure out what was happening and had almost given up on them hatching. As a result only 9 larvae were recovered on the day of hatching. Eight of these were reared to the juvenile stage.
The second spawn did not go much better. I waited until the ninth day to take the eggs, and did not take as many off the rock this time. However, a temperature drop in the aquarium of about 3 degrees C caused a delay in development and hatching for several days and the larvae did not appear until the night of the eleventh day after spawning. I also learned that an unattached egg has greater difficulty hatching than an attached one. As superbly illustrated in Dr. Allan’s book on page 249, Amphiprion eggs hatch tail first. There is a tendency for a larvae hatching from an unattached egg to retain the egg capsule over the anterior portion of the body and swim about pushing the capsule before it. When this happens the larva soon succumbs to oxygen deprivation. Although it was possible to give the hatching larva an assist with a couple of pins, it was not a practical method of getting good larvae for rearing. An estimated 50 larvae were recovered from this spawn and 43 made it through the larval stage. The majority of these were recovered from the breeding aquarium after hatching by locating and concentrating the larvae with a flashlight and siphoning them from the tank. Undoubtedly some were injured by this treatment, but at least 90% were recovered without injury.
The third spawn hatched in 8 days and recovery was entirely accomplished by siphoning the larvae from the breeding tank after hatching. Many larvae were recovered but still only an estimated one fifth of the entire spawn was captured. Immediately after hatching the larvae swim rapidly and apparently without direction for several minutes, and if during this period they encounter the gravel bottom of the tank or the inside of a shell or other restricted area, they probably never survive to the free swimming stage. The total number taken from the aquarium from this spawn was estimated at 125, and a total of 115 were taken from the larval tank in the post larval stage. Thus recovery after hatching was improving and survival rates remained high. Great things were expected from the next spawns for most of the problems had been identified, if not solved, and confidence was high.
Mother Nature struck again. The fourth, fifth and perhaps the sixth spawns were complete wipe outs. All went well until the fifth day after spawning. At this time I noticed a whitening of the eggs and close examination revealed a granular whiteness of the yolk. The embryo appeared normal, but stunted, and hatching never occurred. When the same thing happened on the fifth spawn, I took some of the infected eggs to a friend At the Florida Department of Natural Resources Marine Research Laboratory to take a look at them through the microscope and see if we could determine the causative agent. We did find some “bugs” in the eggs in large numbers and made a very tentative identification putting them in the microsporida. If such is the case, the infection is probably located in the ovary of the female and may have been introduced with some food item, although it is possible that it entered with the natural sea water or was already in the fish in another developmental stage before she spawned the first time. This type of infection does yield to the SSA treatment (Sterilize and Start Again) so future experimentation must wait until new set ups are prepared. This problem can be avoided, unless it is inherent in all female Amphiprion through careful processing of water and food to prevent introduction.
Larval Rearing
An early post larval clownfish about 8 mm long and 10 days old. The orange color is well developed and the white nape stripe is formed across the dorsal surface. The central white band has yet to form.
Good success was obtained in rearing the larvae from the above reported spawns. Survival to the post larval stage was estimated in the second and third spawns, but could not have been less than 60% and was probably closer to 80%. The post larval stage was entered when the fish became 1/4 inch long, had developed the orange body color, had formed the white stripe at the nape, and began thigmotaxis (association with a solid object). At this point the fish could be netted and transferred to a grow-out aquarium.
The larval tanks were rather small, 36 gallons, and were designed to provide the proper environment for the newly hatched larvae. Temperature, lighting, salinity, and water quality were all considered in establishing the larval tanks. The design of the tanks also allowed development of water currents and micro-turbulence that aided floatation of food and larvae. The larvae were fed all live foods while in the larval tanks. Specially processed wild plankton and cultures of micro-organisms were the first food and after three days the larvae were large enough to accept brine shrimp and particulate dead and dried foods.
Growth of the larvae was quite fascinating. The disparity of growth rates among larvae from the same spawn that apparently had equal opportunity to feed was quite large. A few of the larvae entered the post larval stage within 5 days, the majority were at this point of development in 8 days and a few took 15 to 18 days before they gained color and abandoned their pelagic mode of life. This great variance in larval growth rate may be more pronounced in nature and may have bearing on distribution of Amphiprion in the Indo-Australian Archipelago- Philippines region. Those larvae that pass quickly through the larval stages would settle near the place of spawning while those with an extended larval life may settle far from their place of origin. There was no apparent difference in viability between the fast and slow growing larvae.
Juvenile Growth

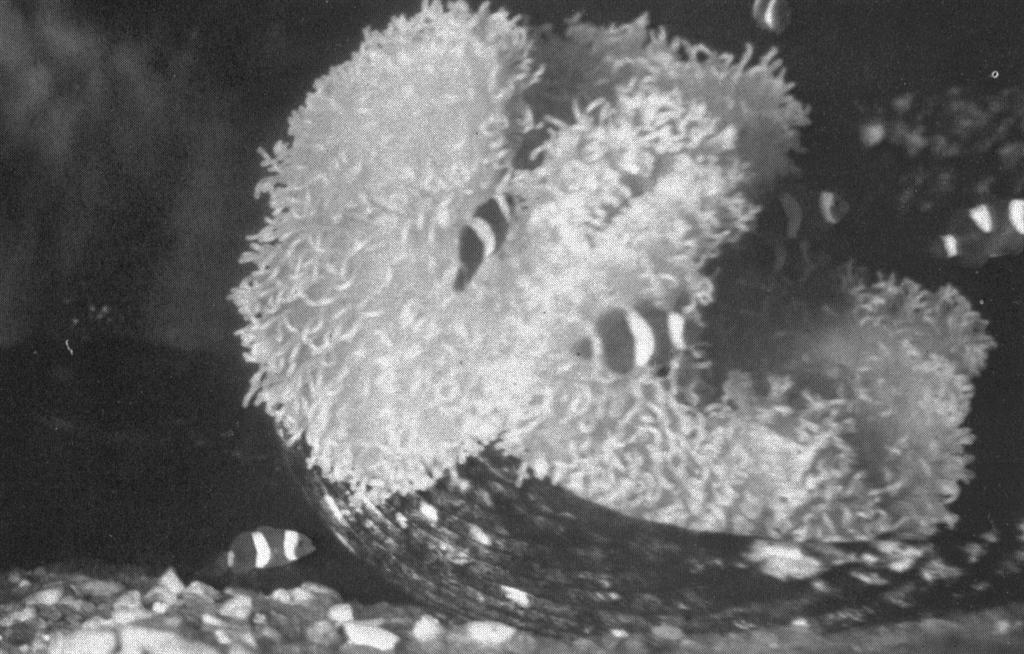
First photo ever of tank raised clownfish! A gathering of small clownfish in the large 60 gallon tank. These fish are about two months old. Several with incomplete mid-body white bands can be seen. All of these and more were hatched and reared by Martin A. Moe. Who said it couldn’t be done? Photo by Martin A. Moe, Jr.
When the majority of the larvae reached the post larval stage, about 10 days after placement in the larval rearing tank, they were removed and placed in an established 20 gallon aquarium to gain in size before being transferred to a 60 gallon grow out tank. At this time they were fed a formula similar to that fed to the adults but processed so that it would break up into many fine particles upon introduction to the tank. Newly hatched brine shrimp were also fed. The fry reached lengths of ½ inch in three weeks and many are about an inch long at an age of 6 weeks. There are no signs of malformations due to malnutrition or any parasitic infections. The fry are very alert and active and even began intraspecific behavioral interactions in the early post larval period. There was one small anemone in the first juvenile tank and the newly introduced post larvae quickly (within two hours) occupied the anemone. I did not observe any period of acclimation by the small fish to the anemone. There was no noticeable avoidance reaction by the fish when it first touched the anemone, however, the post larvae spent up to an hour drifting about the edge of the anemone before entering among the tentacles.
Eight to ten of the larger post larvae established territories on the face of the anemone and guarded this area vigorously. The largest, most dominant fish occupied the center of the anemone. At night these territories disintegrated and at least 50 small clownfish would enter the anemone and nestle among the tentacles. An interesting coloration abnormality occurred in about 10% of the fish. The center stripe, which forms at about 10 to 15 days of age, does not form completely. In some fish only a dorsal saddle is present and in others the bar is incomplete on one or both sides. One fish, a runt, had only one small oval white spot on the right side instead of a mid-body band. There is a tendency for the abbreviated band to develop toward normalcy as the fish ages, but as of this writing none of the markedly brief bands have become completely normal. This unusual banding usually occurs among the fastest growing fish, although there are exceptions. It may be that the period of band formation is somehow disrupted by the rapid larval growth than the tank reared larvae experience.
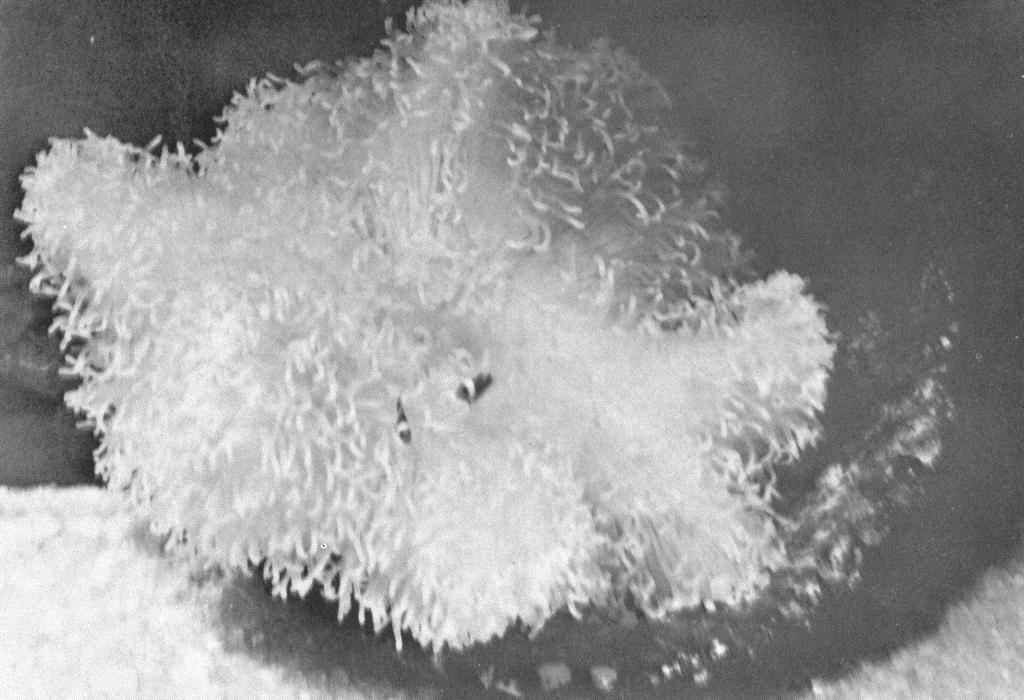
Post larval clownfish in a small anemone. These are about 12 days old and are quite content with their anemone. Six others not clearly visible in the in the photo share the anemone. Behavioral interactions occur frequently between the fish at this age.
The black edging of the pelvic fins are the first black areas to develop. The largest juveniles are now displaying the black edging on the caudal and soft dorsal fin. The white band about the caudal peduncle is the last white band to develop and this is usually in evidence at about 15 days.
Many problems remain to be solved (some haven’t even been identified) before breeding marine tropical fish becomes routine. However, the successes reported in this brief article indicate that production of large numbers of marine tropical fish is possible. The very basic technology has been developed and given the proper facilities and development time, I feel that many species could be cultured. The basic principles are adaptable to other species, certainly to other Amphiprion, but I am sure many specialized problems will be encountered with various species. The fish from these experimental spawns are now on display at Scott’s Highway Aquarium in St. Petersburg, Florida. I hope future articles will be able to recount additional successes and perhaps more details on spawning and rearing procedures.
References
- Allen, G. B. 1972. Anemonefishes, their classification and biology. T.F.H. Publications, Inc. LTD, neptune City, N. J. : 1 – 288 pages.
- Meulengracht-Madsen, J. 1071. Breeding Amphiprion percula. Tropical Fish Hobbyist, Vol. XIX, March 1971: 52-57.
- Neugegauer, W. 1969. So zuchen wir Korallenfische. Aquarien Macazin. Stuttgart, Dec. 1969: 483-488.
- Schreiner, W. 1972. Breeding Report Clownfishes. The Marine Aquarist. Vol. 3, No. 31-33.
Breeding the Clownfish, Amphiprion ocellaris, postscript
The sixth spawn was also infected with the parasite within the eggs, however, only a portion of the spawn was lost. The eggs were removed from the parents a few hours after spawning and were treated in a solution of sulfathiazole and quinine for three hours. They were then artificially incubated in a methylene blue solution for the entire 8 days of development. The presence of the parasite was noted on the evening of the fourth day of incubation and on the fifth day 330 infected eggs were removed from the rock. Four more were removed the following day and the remaining eggs seemed to develop normally. About 200 eggs remained and hatching took place on the evening of the eighth day. Not all the eggs hatched, about 50 remained on the rock and were expected to hatch the following night. At this point, I made an error. The tank was not adjusted to the larval rearing condition because the remaining eggs were still incubating and about 50% of the newly hatched larvae were lost. Most of the remaining eggs did not hatch anyway, thus all the trouble and loss of larvae was unnecessary .Good experience was gained, however, and about 50 strong larvae resulted from the sixth spawn. Even in larvae 48 hours old, remarkable size variation occurs. Some of the larvae are already 6 mm long and beginning to develop orange coloration while others are still the size of a newly hatched larva.



0 Comments