
Phosphate is an ion of great concern to reef aquarists. In fact, aside from calcium and alkalinity, it is probably the chemistry topic on which reef aquarists focus the most. Much of this concern is warranted, with phosphate potentially contributing to algae problems, poor coloration of corals and other invertebrates, and growth of most photosynthetic organisms. For these reasons, every reef should have a plan for export of phosphate in one or more ways, and the choices abound. Topics relating to desired target levels and the many export methods have been covered in detail by me and other authors in the past, and I won’t dwell on them here.
What I will focus on relates to the various sources of phosphate in reef aquaria, and how important each one actually is to an operating aquarium. There have long been numerous misunderstandings of these source issues, but in the last two years it seems that these misunderstandings have become more common and are sometimes driving aquarists to unwarranted actions.
In part, I attribute this recent uptick in confusion to the newly available phosphate checkers from Hanna. Without getting into any discussion of their relative accuracy or the merits of using them, I think many aquarists are being lured into inappropriate actions by finding phosphate in various additives that they were not aware of previously. To paraphrase Field of Dreams: “Build it and they will find phosphate”. In order for aquarists to interpret what these found values mean, they need to have an understanding of the overall phosphate balance in coral reef aquaria. This article strives to provide the understanding necessary to put phosphate issues into proper perspective.
So just for starters, let’s see how many of you would be concerned by each of these scenarios:
- My purified fresh water I use for top off has 0.05 ppm phosphate in it. Since I’m trying to keep my tank at 0.02 ppm, that’s obviously too much. What should I do?
- I put a cube of my frozen fish food in a half cup of water and did a phosphate test on the water. I got a whopping value of 1.0 ppm!!! That’s a huge problem, right?
- I put a teaspoon of my GAC (granular activated carbon) in a glass of fresh water, and then tested the water for phosphate. It was dark blue and I could not even get a reading there was so much. Time to look for another brand, right?
- I know I feed my fish and corals, but I carefully rinse my foods, use only frozen foods, and only feed enough that all is eaten. So I have no idea where my phosphate is coming from. Must be my RO/DI isn’t working as there is no other possible source.
Experience tells me that 95% or more of reef aquarists agree that the first three are problems to be solved, and at least half have the same feelings as expressed in number four. In reality, the first three are really not substantial issues at all and reefers need to understand why. Scenario four sums up the main point reefers need to understand: foods are the primarily source of phosphate in almost any aquarium that is being fed, regardless of the choice of foods and rinsing etc. In most aquaria, this source dominates all other sources by a factor of ten to a hundred or more, even if scenarios 1-3 are also true!
Food Sources of Phosphate
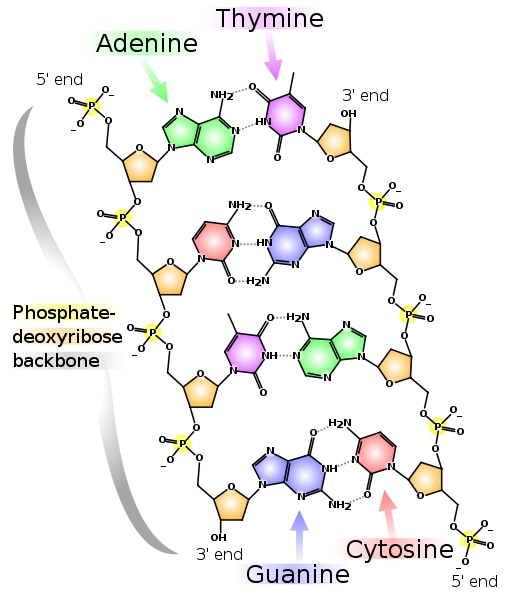
In order to begin to understand phosphate sources in aquaria, let’s first look at foods. Contrary to what many suppose, phosphate is not something that can be avoided in a nutritious fish food. Phosphorus is present in many of the biomolecules of life, so every natural tissue that goes into a fish food will have substantial phosphorus in it. Phospholipids make up a substantial part of cell membranes. The genetic code of every organism is made of DNA, and that DNA contains a phosphate bridge between each base pair (Figure 1 shows how much phosphate is really in DNA). The way that all cells get energy is through conversion of ATP to ADP, and these molecules contain 3 and 2 phosphate moieties respectively (Figure 2).
Most importantly for nutritional aspects of foods and phosphate, proteins contain phosphate. Many budding chemists understand that proteins are made of amino acids, and none of the standard amino acids contain phosphate, so where does the phosphate come from? It turns out that organisms attach phosphate to the hydroxyl group of the amino acids threonine and tyrosine in proteins to turn on and off many types of protein functions. Consequently, proteins often contain a lot of phosphate, making it very hard to make nutritionally complete foods without substantial phosphate. The relationship between phosphate and protein is so tight that people who suffer from excess phosphate (typically those with kidney disease, and especially those on dialysis) are unable to get enough protein without getting too much phosphate. These patients take oral phosphate binders to bind dietary phosphate before it is absorbed from their small intestines because restricting dietary phosphate just is not effective enough.
Quantitation of Phosphate in Foods
How much phosphate is in foods? A lot, but it does depend on the food. Some aquarium foods state on the label a minimum specified amount of phosphorus (phosphorus is the atom, P, at the center of a phosphate ion, PO4—). Analyses of foods often quote an amount of phosphorus instead of phosphate as the unit of measure. Like feet and inches, these are just different units of measure of the same thing, and values of phosphorus are multiplied by 3.1 to get values to phosphate.
For foods that are intended for human consumption (such as shrimp or clams), we can look up known data on phosphorus content. Unfortunately, many manufacturers of aquarium foods do not supply phosphorus values and they are complex mixtures of many ingredients which preclude us from looking them up. Perhaps some manufacturers are concerned about scaring aquarists about the phosphate content. Fortunately, Ron Shimek analyzed a variety of foods several years ago and this data set can also be used to understand how much phosphorus is in various commercial aquarium foods. Tables 1-3 contain data on the phosphorus and protein content of many aquarium foods, with Table 1 containing dry foods, Table 2 containing frozen foods, and Table 3 containing grocery store foods that are sometimes used in aquaria.
These raw phosphorus values alone allow us to know how much phosphorus is entering the aquarium by feeding a certain food, and we will use these numbers in several analyses later in this article. One must be wary of relying too much on such numbers for comparative purposes, however, since some foods have substantially more moisture or fillers in the food, which serve to drop the percent phosphorus, but are not necessarily better choices from a phosphorus standpoint. One simply needs to feed more of a wetter food than a drier one to attain the same total nutrition, offsetting the lower claimed phosphorus value.
One useful way to compare foods to each other is by comparing the phosphorus to protein ratio. In this crude way, we can eliminate effects due to moisture and fillers. Obviously this method has its own flaws since the nutritional value of foods is far more complex than a protein value alone provides, but it will allow us to understand among similar types of foods, which ones seem to provide more or less phosphorus. In order to help guide the reader, I have chosen to show the higher phosphorus to protein foods in red and the lower ones in green in Tables 1-3. The cutoff values I used are completely arbitrary, and one should definitely not make a choice of food based on this criterion alone. Nevertheless, several points are apparent:
- Dry and frozen foods vary substantially in phosphorus to protein content. There are high and low phosphorus foods in both categories. On balance, one could not say from this data that typical dry foods are any worse in this regard than are typical frozen foods.
- With the exception of seaweeds, grocery store foods do generally seem to have a lower phosphorus to protein ratio than many other choices, although several Ocean Nutrition frozen foods are similarly low. This presumes that one is using grocery store foods that are not treated with phosphate to preserve freshness in the processing plant, but that is a concern for many of these materials.
- Shrimp seem to be a standout in terms of a low protein to phosphorus ratio for grocery store foods.
- Fish with bones have a high phosphorus content, since bones are modified calcium phosphate, but whether these bones are fully digested or not probably depends on what eats them and how long one otherwise waits for them to decompose.
| Food | Protein (mg/g) | Phosphorus (mg/g) | Phosphorus/Protein(w/w) | Source of Data |
|---|---|---|---|---|
| Brine Shrimp Direct Golden Pearls | 550 | 15 | 0.027 | Shimek |
| Brine Shrimp Direct Plankton Gold Flakes | 490 | 8.3 | 0.017 | Shimek |
| Cobalt Aquatics Brine Shrimp Flakes | 440 | 10 | 0.023 | Label |
| Cobalt Aquatics Spirulina Flakes | 450 | 10 | 0.022 | Label |
| IO Marine Chips Herbivore | 460 | 9 | 0.020 | Label |
| IO Marine Chips Omnivore | 460 | 11 | 0.024 | Label |
| IO Marine Grazing Block | 40 | 0.5 | 0.013 | Label |
| IO Marine Pellets Herbivore | 440 | 10 | 0.023 | Label |
| IO Marine Pellets Omnivore | 470 | 12 | 0.026 | Label |
| IO Seaweed Grazing Blocks | 25 | 0.3 | 0.012 | Label |
| Nutrafin Max Marine Angel Sinking Pellets | 440 | 8 | 0.018 | Label |
| OSI Marine Flake | 470 | 6 | 0.013 | Label |
| TetraAlgae Vegetable Enhanced Crisps | 460 | 10 | 0.022 | Label |
| TetraMarine Flakes | 460 | 12 | 0.026 | Label |
| TetraMarine Granules | 440 | 14 | 0.032 | Label |
| Vibragro Saltwater Staple | 300 | 15 | 0.050 | Shimek |
| Food | Protein (mg/g) | Phosphorus (mg/g) | Phosphorus/Protein(w/w) | Source of Data |
|---|---|---|---|---|
| Direct Tahitian Blend Cryopaste | 44 | 1.4 | 0.032 | Shimek |
| Frozen Plankton/Krill Brine Shrimp | 88 | 1.6 | 0.018 | Shimek |
| Gamma Foods Lancefish | 180 | 4.4 | 0.024 | Shimek |
| Hikari Bio-Pure Clam On A Half Shell | 32.9 | 3 | 0.090 | Label |
| Hikari Bio-Pure Clam On A Half Shell (meat only) | 128.4 | 4.1 | 0.032 | Label |
| Ocean Nutrition Frozen Formula 1 | 160 | 1.1 | 0.007 | Shimek |
| Ocean Nutrition Frozen Formula 2 | 62 | 1.2 | 0.019 | Shimek |
| Ocean Nutrition Frozen mysis | 52 | 0.1 | 0.002 | Label |
| Ocean Nutrition Frozen Prime Reef | 130 | 0.9 | 0.007 | Shimek |
| Oregon Desert Brine Shrimp Company Silversides | 42 | 4 | 0.095 | Shimek |
| San Francisco Brand Frozen Brine Shrimp | 31 | 0.72 | 0.023 | Shimek |
| Food | Protein (mg/g) | Phosphorus (mg/g) | Phosphorus/Protein(w/w) | Source of Data |
|---|---|---|---|---|
| Broccoli | 30 | 0.66 | 0.022 | analysis |
| Clams (no shell) | 128 | 1.69 | 0.013 | analysis |
| Clams (no shell) | 128 | 1.69 | 0.013 | analysis |
| Cod | 179 | 1.74 | 0.010 | analysis |
| Cod | 229 | 2.23 | 0.010 | analysis |
| Eel | 184 | 2.16 | 0.012 | analysis |
| Eel | 186 | 2.16 | 0.012 | analysis |
| Kelp | 17 | 0.42 | 0.025 | analysis |
| Mackerel | 186 | 2.17 | 0.012 | analysis |
| Mussel | 119 | 1.97 | 0.017 | analysis |
| Nori | 290 | 6.4 | 0.022 | Shimek |
| Octopus | 149 | 1.86 | 0.013 | analysis |
| Oyster (no shell) | 70 | 1.35 | 0.019 | analysis |
| Oyster (no shell) | 71 | 1.35 | 0.019 | analysis |
| Salmon | 204 | 2.4 | 0.012 | analysis |
| Sardine | 209 | 3.66 | 0.018 | analysis |
| Scallops | 168 | 2.19 | 0.013 | analysis |
| Shrimp | 209 | 1.36 | 0.007 | analysis |
| Shrimp | 210 | 0.7 | 0.004 | analysis |
| Spirulina | 59 | 1.1 | 0.019 | analysis |
| Squid | 156 | 2.2 | 0.014 | analysis |
| Wakame (seaweed) | 30 | 0.8 | 0.027 | analysis |
Impact of Foods on the Aquarium Phosphate Balance
Now we come to the heart of the issue. The actual amount of phosphorus present in foods and what it means. In order to understand the effects of foods, we need to understand what happens to them when added to an aquarium. Some aquarists are under the misconception that eaten foods do not contribute to the free phosphate in the water. Many aquarists are told the mantra of feeding only as much as is eaten, and they confound this idea with the assumption that when doing so, one minimizes the phosphate release. That idea is simply untrue.
A fish or other organism that eats foods takes in substantial phosphate, as shown above. But what happens to it? If the organism is not actually expanding in size (such as an adult green chromis, or a person), the phosphate that is taken in is almost entirely excreted back into the water. The only exception to that process is the very small amount of phosphorus that goes into eggs or sperm, and since in most aquaria those items are rapidly consumed by other organisms, the phosphorus will ultimately get into the water.
Growing organisms do take up a small amount of phosphorus from the diet and retain it in their growing tissues, but the emphasis is on small. A study of a fish farm with rapidly growing rainbow trout in the ocean showed that 78-82% of the phosphorus feed to the fish was lost to the environment. A second aquaculture study using normal fish foods showed that 62% of the fed phosphate was released to the environment, with 35% being released as soluble phosphate available directly to algae, and 27% as phosphorus in fecal pellets (which if not removed, will break down in an aquarium releasing the phosphate again). Another study showed that 81.5% of commercial diet phosphate was released to the environment, but that with a “special” diet with low phosphate and low fish meal this could be reduced to 64% lost. A fourth study showed that growing fish fed slightly less phosphate than they need (to optimize theoretical uptake) take up and retain different phosphate sources differently. Using a purified protein diet, they observed retention of 72% of the phosphorus, 51% retention of phosphorus from added fish bone meal, and higher levels of uptake and retention for inorganic phosphate supplements (such as sodium phosphate).
This sort of study is of concern in aquaculture settings due to environmental contamination due to the released phosphorus and nitrogen. To my knowledge, however, it has never been done in a reef aquarium. Such phosphorus balance studies have also been performed in people for many years. In adults it is clear that nearly all phosphate taken up is excreted, mostly in the urine and some in the feces. Even in young growing children, the amount of phosphorus retained from the diet is only 5-20% of that consumed, with 80-95% excreted in the urine and feces. While such studies are fairly far removed from reef aquaria, they do supporting the idea that organisms take in a lot more phosphorus than they retain, even when growing.
Consequently, reef aquarists should expect that much of the phosphorus added to a reef aquarium in the form of foods ultimately ends up in the water as phosphate. Whether that portion getting into the water is 95% or 35% won’t substantially impact the conclusions below that foods add a very large amount of phosphate.
Quantitation of the Food Impact Aquarium Phosphate
Using the assumption that most of the phosphorus present in foods ultimately ends up as phosphate in the aquarium, we can calculate roughly what that effect is. Even if the actual number is a half or a quarter of that added, getting the ballpark information is very useful to gauge the importance of this phosphate source. Obviously the calculated value depends on how much of what is fed to what size aquarium.
Table 4 shows a variety of possible feeding schemes that an aquarist might use on a hypothetical 100 gallon (379 L; actual water volume) aquarium. Aquarists can decide for themselves how these regimens compare to their own feed schedules.
| Foods Fed | Phosphorus Added Daily (mg) | Equivalent Phosphate Added Daily (mg) | Equivalent Phosphate Concentration Added Daily (ppm) |
|---|---|---|---|
| 1 Prime Reef Cube | 2.7 | 8.4 | 0.022 |
| 1 Prime Reef Cube1 Formula 1 Cube | 6.0 | 18.6 | 0.049 |
| 1 Formula 2 Cube1 Mysis Cube | 3.9 | 12.1 | 0.032 |
| IO Marine Omnivore Chips (2 g) | 22 | 68 | 0.18 |
| IO Marine Omnivore Chips (1 g)Silversides (1/2 teaspoon)Nori (2.5 g = large sheet) | 37 | 115 | 0.30 |
Obviously there is a big range depending on how much is fed. What is surprising to many folks, however, is how large that number is relative to typical target levels of phosphate in reef aquaria, which might be something like 0.03 ppm or less. Even the light feeding of a single cube of a relatively low phosphate frozen food to this aquarium supplied most of that target amount in a single feeding. Heavy feeding added ten times that amount in a single day. In short, this high daily addition rate is why phosphate control is often very difficult or reef aquaria.
Rinsing Foods and the Effect on Phosphate
Now that we have some information on the phosphate in foods, we can critically examine the concern that many aquarists have about foods, and specifically their rinsing of frozen foods before use. A typical test you see is someone taking a cube of fish food, thawing it, and putting it into a half cup of water. They then test that water for phosphate and find it “off the charts”. Let’s assume that means 1 ppm phosphate, which would give a very dark blue color in many phosphate tests. Bear in mind this is a thought problem, not an actual measured value, but it is typical of what people think the answer is.
Is that a lot of phosphate? Well, there are two ways to think of the answer.
The first way is as a portion of the total phosphate in that food. A half cup of water at 1 ppm (1 mg/L) phosphate contains a total of 0.12 mg of phosphate. A cube of Formula 2 contains about 11.2 mg of phosphate. So the hypothetical rinsing step has removed about 1 percent of the phosphate in that food. Not really worthwhile, in my opinion, but that decision is one every aquarist can make for themselves.
The second way to look at this rinsing is with respect to how much it reduces the boost to the aquarium phosphate concentration. Using the same calculation as above of 0.12 mg of phosphate, and adding that to 100 gallons total water volume, we find that phosphate that was rinsed away would have boosted the “in tank” phosphate concentration by 0.12 mg/379 L = 0.0003 ppm. That amount washed away does not seem significant with respect to the “in tank” target level of about 50-100 times that level (say, 0.015 to 0.03 ppm), nor does it seem significant relative to the total amount of phosphate actually added each day in foods (which is perhaps 50-1000 times as much, based on input rates from Table 4. Again, the conclusion I make is that rinsing is not really worthwhile, in my opinion.
Comparison of Food Sources of Phosphate to Other Sources
What about other sources of phosphate, like the “crappy” RO/DI water containing 0.05 ppm phosphate? A similar analysis will show it equally unimportant relative to foods.
Let’s assume that the aquarist in question adds 1% of the total tank volume each day with RO/DI to replace evaporation. Simple math shows that the 0.05 ppm in the RO/DI becomes 0.0005 ppm added each day to the phosphate concentration in the aquarium. That dilution step is critical, taking a scary number like 0.05 ppm down to an almost meaningless 0.0005 ppm daily addition. Since that 0.0005 ppm is 40-600 times lower than the amount added each day in foods (Table 4), it does not seem worthy of the angst many aquarists put on such measurements. That said, tap water could have as much as 5 ppm phosphate, and that value could then become a dominating source of phosphate and would be quite problematic. Purifying tap water is important for this and many other reasons.
The same sort of calculation applies to analyzing other phosphate issues, such as the GAC in scenario three. The issue of finding “high” phosphate in GAC soaked in fresh water was frequently quoted as a reason to use one or the other brand of GAC, and probably still is. But simple analysis as shown above for the food rinsing puts the lie to this being a big problem.
One needs to consider how much GAC one will really use in the aquarium and how often it is added in order to interpret how important the added phosphate is. A typical recommendation might be 1 cup of GAC per 100 gallons of aquarium water, and to change it in 4-6 weeks. Let’s assume we detect 0.5 ppm phosphate when a teaspoon is placed in a cup of water, and we get scared by the dark blue color during the test. Is this reasonable? That 0.5 ppm from a teaspoon in a cup of water translates to 0.015 ppm phosphate when a cup is used in 100 gallons.
That 0.015 ppm may be significant, being a typical target concentration level for reef aquaria and amounting to about half to a twentieth of the amount added daily in foods, but remember, it is used for 4-6 weeks. During those 4-6 weeks before the next replacement, foods add 50-700 times as much phosphate. So while it is not unreasonable to look for another brand of GAC, to blame phosphate or algae issues in the aquarium on its use would stretch credibility because it is a very tiny portion of the total phosphate being added.
Conclusion
Foods are by far the most important source of phosphate in most aquariums. While there are big variations between foods, it does not appear in this analysis that dry foods are the nasties they are often made out to be, relative to frozen foods. There are better and poorer choices (with respect to phosphate) to be made within each food type. Avoiding foods with bones, however, might be worthwhile if delivering less phosphate is a goal. Additionally, fresh grocery store shrimp seems to be one of the best foods from this standpoint.
In considering whether sources of phosphate other than foods are important, one must carefully look to the actual amounts involved to determine whether other sources are even worth trying to minimize. It can be scary to learn that your purified fresh water has phosphate in it, or that your salt mix has detectable phosphate, or that your supplements or whatever have some phosphate. But just because you detect something, and maybe you even detect a concentration far higher than in your aquarium, that does not by any means imply that those sources are significant enough to warrant some sort of corrective action. Our analytical tools have become fairly sensitive, allowing us to detect things which might sound like trouble, but really aren’t. We need to understand the various dilution issues involved as well as the overall phosphate balance in a reef aquarium to evaluate the importance of different measurements.
Just use some math and put it all into perspective, before using some dollars or time to chase a trivial “problem”.
Happy Reefing




My corals are not growing; they are suffering from the negative effects of phosphate in the tank. How do I get them out of my tank?
Wonderful article. Thank you for the time and care that went into creating this!
Fantastic eye opener. Thank you