Abstract
 Coral reefs are rapidly disappearing as carbon dioxide emissions, cyanide fishing, and eutrophication increase. Antithetically, the reef aquarium trade has experienced an increase of demand, although many aquarists have an absent understanding of seawater chemistry and coral physiology. In this study, the health of three hermatypic coral species (Caulastrea furcata, Pavona decussata, and Pocillopora damicornis) were analyzed via PAM fluorometry in hostile water conditions. The calcium, alkalinity, and pH parameters were altered to match ocean acidification conditions and extreme low and high values that are reached due to improper dosing in an aquarium. Among the species examined, the maximum potential quantum yield (Fv/Fm) was significantly affected in hostile conditions. Specifically, a decline in health was observed in elevated alkalinity and in decreased calcium conditions. Furthermore, corals did not exhibit a negative response in Fv/Fm during low pH conditions.
Coral reefs are rapidly disappearing as carbon dioxide emissions, cyanide fishing, and eutrophication increase. Antithetically, the reef aquarium trade has experienced an increase of demand, although many aquarists have an absent understanding of seawater chemistry and coral physiology. In this study, the health of three hermatypic coral species (Caulastrea furcata, Pavona decussata, and Pocillopora damicornis) were analyzed via PAM fluorometry in hostile water conditions. The calcium, alkalinity, and pH parameters were altered to match ocean acidification conditions and extreme low and high values that are reached due to improper dosing in an aquarium. Among the species examined, the maximum potential quantum yield (Fv/Fm) was significantly affected in hostile conditions. Specifically, a decline in health was observed in elevated alkalinity and in decreased calcium conditions. Furthermore, corals did not exhibit a negative response in Fv/Fm during low pH conditions.
Introduction
Hermatypic (reef building) corals are rapidly disappearing due to dredging, cyanide fishing, eutrophication, overharvesting, and increasing sea surface temperatures (Edinger et al. 1998, Hughes et al. 2003, Marubini and Thake 1999). However, to fully understand the direct biochemical effect on corals, the anatomy and physiology of these corals must also be understood.
All hermatypic corals are made of an aragonite skeleton which is a crystalized form of calcium carbonate (CaCO3) (Barnes 1970). With the continuous increase of carbon dioxide into the atmosphere, the ocean has experienced an increase of carbonic acid. Carbonic acid inhibits the exchange of hydrogen ions which eliminates the production of calcium carbonate and decreases pH (Marubini and Atkinson 1999). Without the production of calcium carbonate, coral skeletons weaken and in time degrade completely (Kleypas and Langdon 2000). This process is has been linked to the lack of recovery in coral reefs that have been exposed to dynamite and cyanide fishing practices (Hilbertz 1981 and Jones et al. 1999).
Zooxanthellae (Frudenthal, 1962) have a mutualistic symbiotic relationship with their hermatypic hosts; in return for habitat and nutrients, the zooxanthellae provide photochemically useable energy for the coral, thus accounting for a large portion of a coral’s net energy (Knowlton and Rohwer 2003 and Muscatine and Porter 1977). When water quality reaches unsuitable levels or temperatures become too high, the coral host discontinues the relationship by abandoning, or releasing, the zooxanthellae from its tissues (commonly known as “bleaching”). Coral bleaching has intensified throughout the years and has been the leading cause for the deaths of reefs; including the largest reef in the world, the Great Barrier Reef (Brown 1997 and McCowan et al. 2012). Bleaching has also been observed in aquaria much like a natural reef when water quality is poor or if the temperature is allowed to become suboptimal (Donowitz 2002).
The biodiversity of coral reef habitats has driven an increased interest in tropical reef aquaria across the world (Jones et al. 1999). With advancing husbandry technology (e.g. wave makers, light structures, and mechanical filtration systems), reef aquaria have become easier to own and maintain. With this advancement of mechanical components, there has also been the advancement of water treatment. Aquarium supply companies, such as Seachem and Kent Marine, are bettering chemical treatment for optimal water quality specifically for coral health. The most common water quality parameters tested for and treated within a reef aquarium are alkalinity, calcium, and pH (Donowitz 2002). In order for corals to thrive, these parameters must be maintained at certain levels to create a balanced seawater solution. Natural seawater typically hasan alkalinity of 2.5 meq/L, a pH of 8.2, and a calcium of 410 ppm (Holmes-Farley 2005). In reef aquaria, these parameters are kept at higher levels; alkalinity=3.0-3.5 meq/L, pH=8.2-8.4, and calcium=420-450 ppm. The upkeep of these parameters is maintained via “dosing”, and are often divided into two parts: 1) a calcium chloride solution with added trace elements and 2) a bicarbonate and carbonate solution (alkalinity). When dosed improperly, these parameters can cause an increase of supersaturation, increasing the likelihood of CaCO3 precipitation; also known as “crashing the system” (Holmes-Farley 2005). The precipitation of CaCO3 can be detrimental to corals in a closed system such as a reef aquarium.
When evaluating coral health, the few methods that are currently available are expensive and can come with high error. Littman et al. (2008) compares the accuracy of a hemocytometer to a flow cytometer; measuring the densities of free living zooxanthellae. Many are beginning to adopt this hemocytometer method, which consists of scraping the tissue off of a coral, preserving it in a formalin solution, and then counting the zooxanthellae cells that detached from the tissue (Suwa et al. 2008).
However, a newer and less invasive method called PAM chlorophyll fluorometry has been favored and used since the early 1990’s (Jones et al 1999, Suwa et al. 2008, and Kramer et al. 2012). This method provides a measurement of maximum potential quantum yield (Fv/Fm), which is the variance of minimum and maximum fluorescence divided by the maximum fluorescence. In short, the fluorometer measures how efficiently the zooxanthellae convert light into photochemically usable energy for the coral (Jones et al 1999, Suwa et al. 2008, Kramer et al. 2012, McCowen et al. 2012, and Zartler 2012). This method can be applied in a home aquarium using the smaller and less expensive Heinz Walz Jr. PAM chlorophyll fluorometer (Zartler 2012).
Within this experiment I analyzed the health of three hermatypic coral species (Pavona decussata, Pocillopora damicornis, and Caulastrea furcata) was analyzed via PAM fluorometry in hostile water conditions. Calcium, alkalinity, and pH were altered to match (1) scenarios in relation to ocean acidification and (2) extremely low and high potential values reached in an aquarium due to improper dosing. The purpose of this study is to understand the direct effects of poor (or hostile) water quality has on hermatypic corals in a captive environment and to better understand the potential impacts of ocean acidification on these corals.
Methods
Experimental Design
Seven 5-gallon aquariums were positioned 7 cm apart on a cabinet top in the Biology Lab at Alaska Pacific University (APU). Each aquarium was labeled in accordance to a specific experimental treatment (Table 1). A plastic container was placed in each tank and filled with live sand and amphipods that were previously cultured in a well-established aquarium for biological filtration. The live sand was oxygenated with an air pump to support proper circulation. Additionally, hang-on-back filters were set up on each system containing a filter sponge and activated carbon for mechanical and chemical filtration. Water quality was maintained at natural seawater conditions by applying weekly partial water changes (10 %) using Red Sea Salt mix, until treatments began.
Collection of Specimens
Fragments of the three hermatypic coral species, C. furcata, P. decussata, and P. damicornis, were clipped with bone cutters from their parent colonies that have been long established in a display tank in APU’s Aquarium Lab. Twenty-one fragments of each species were prepared (average length of 5 cm). Individual fragments were cut specifically by species: 2-3 polyps for each C. furcata, 1-2 “chips” for each P. decussata, and 2-3 branches for each P. damicornis (Figure 1). Once the corals were fragmented, each set of polyps, chips, and branches was placed on a 3 cm long ceramic plug and attached using cyanoacrylate adhesive. The fragged corals were then uniformly distributed and acclimated into seven different experimental tanks (three frags of each species per tank). All tanks were cultured 2 months prior to the time of acclimation.

Figure 1. Coral fragments of the three species C. furcata (left), P. decussata (middle), and P. damicornis (right).
Water Quality Manipulation
A 33% solution of Hydrochloric acid (HClaq) was used to reduce and maintain a low pH of 7.9 in the “Low pH” system. This was achieved by dosing 0.25 mL of HClaq every 10 minutes for ~2-3 hours, 3 times a week for one week prior to the fluorometry sampling dates. The “High pH” system was dosed with a 42 % solution of Limewater and maintained a high pH of 8.35-8.40. The dosing procedures were the same as those of the low pH.
The “Low Alkalinity” system was kept at 1.5 meq/L by dosing 0.625 grams of Seachem’s Reef Builder calcium additive 4 times every 10 minutes, 1-3 times a week, given the previous week’s water quality results. A calcium additive, Seachem’s Reef Carbonate, was used to decrease alkalinity because of the negative relationship of the two parameters in a reef aquarium (Seachem Laboratories Inc 2004. In order to achieve and maintain a high alkalinity of 7.0 meq/L, 0.6 grams of Seachem’s Reef Carbonate alkalinity additive was dosed 4 times to the “High Alkalinity” system every 10 minutes, 3 times a week.
The “Low Calcium” system was maintained at 350 ppm by dosing 0.6 grams of Seachem’s Reef Carbonate additive 4 times, every 10 minutes 1-3 times a week (based on the previous week’s water quality results). The “High Calcium” system was maintained between 500- 600 ppm, by dosing 0.625 grams of Seachem’s Reef Builder calcium additive, 4 times every 10 minutes, 3 times a week.
Water Quality Analysis
Water quality was tested using Seachem’s Marine Basic test kit (Seachem Laboratories Inc., 2004), which measures the following parameters: pH, alkalinity (meq/L), nitrite and nitrate (mg/L), total (or ionized) and free ammonia (mg/L). Every parameter previously listed was assessed. Nitrate and nitrite generally peak when excess nutrients are added to a system which could cause stress among corals. It was important to maintain these at 0.00 mg/L during the entire duration of this experiment (Hughes et al. 2003). Additionally, ammonia is toxic in an aquarium and should also be kept at 0.00 mg/L (Seachem Laboratories Inc. 2004). Temperature was maintained at 26°C for all tanks. Salinities were also measured daily using an optical refractometer and all systems were maintained at a range of 33-34 ppt for the duration of the experiment.
Fluorometry Sampling
Fluorometry sampling followed the methods of Kramer et al. (2013). A Heinz Walz Jr-PAM (Pulse Amplitude Modulated) chlorophyll fluorometer with a 450 nm pulse applied through a 100 cm and 1.5 mm diameter fiber optic cable was used to derive maximum potential quantum yield. Measurements were taken less than 1mm from and parallel to the corals’ coenosarc (polyp- connecting tissue). PAM WinControl-3 software was used when applying saturating pulses.
Statistical Analysis
Multiple regression was used to determine the response of maximum potential quantum yield in relation to the changes of pH, alkalinity, and calcium with a species effect. Due to unexplained deaths, nine P. damicornis fragments were not considered in this analysis. All data was analyzed in the software program “R” (R Core Team 2012).
Results
pH
There was a significant difference in the maximum potential quantum yield among species by pH (F = (3, 328) 15.1, p-value < 0.001), see Figure 2a; however, there was a weak relationship (r2 = 0.121), see Figure 3b. C. furcata and P. damicornis had a significant difference (p-value < 0.05), and P. decussata, showed no significant difference (p-value = 0.143). Among all species there was a noticeable peak in Fv/Fm around 8.2.
Alkalinity
The maximum potential quantum yield showed species-specific responses to changes in the alkalinity (F = (3, 328) 43.1, p-value < 0.001), see Figure 2b. Similar to pH, a weak relationship (r2 = 0.283) was observed (Figure 3a). Additionally, C. furcata and P.damicornis both had a significant difference (p-values < 0.001) in Fv/Fm, however P. decussata did not (p-value = 0.107).
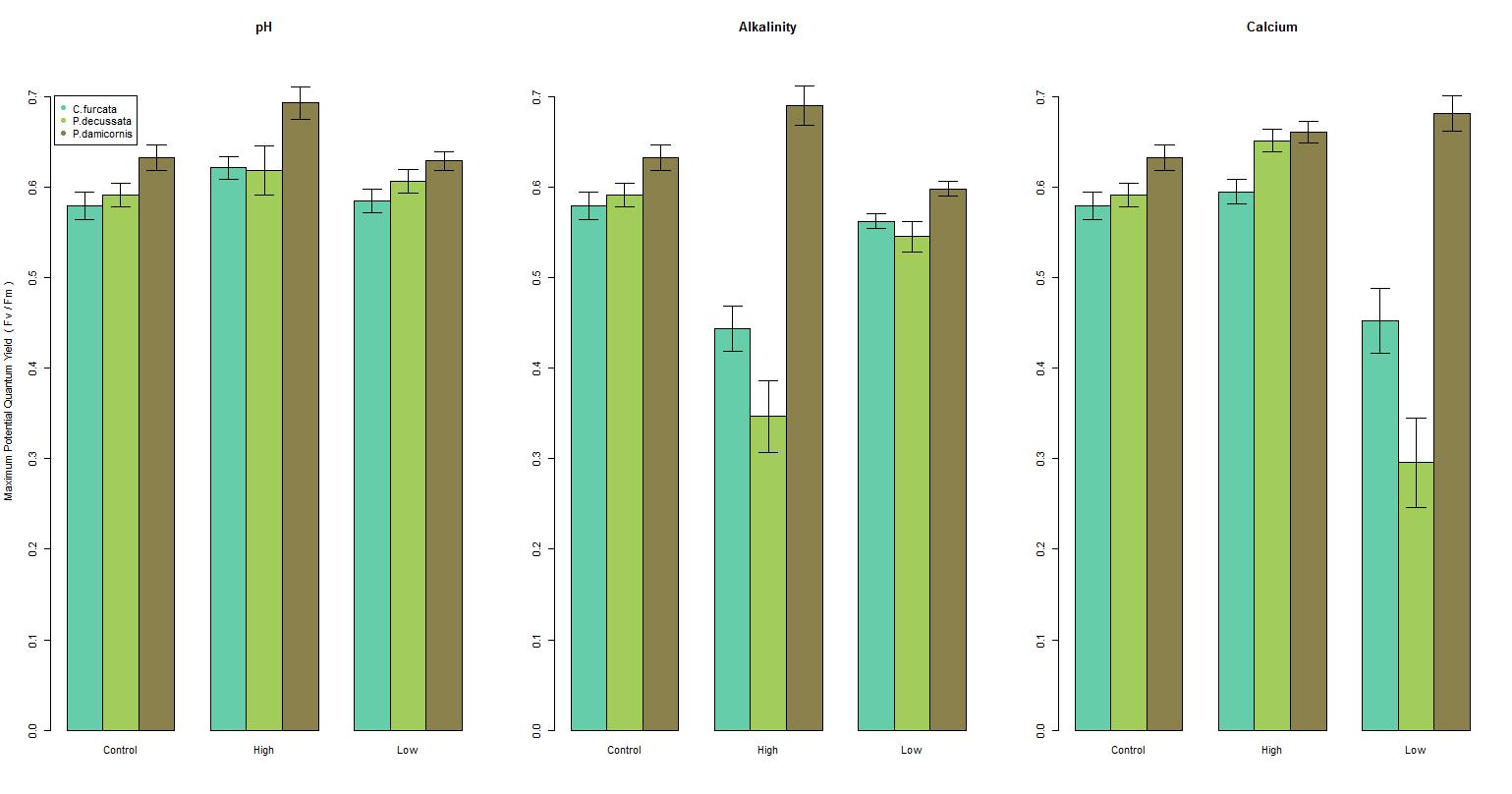
Figure 2. A general overview of the changes of the maximum potential quantum yield by each parameter scenario (high and low) compared to the reference. Sea green represents C. furcata, green represents P. decussata, and brown represents P. damicornis. Each bar is within 2 standard errors of the mean.
Calcium
There was a significant difference in maximum potential quantum yield values among the calcium treatments of the three species (F = (3, 319) 17.0, p-value < 0.001), see Figure 2c. However, similar to pH and alkalinity there was a weak relationship (r2 = 0.134), see Figure 3c. Specifically there was a significant effect among C. furcata and P. damicornis (p-values < 0.001). Again, P. decussata was not significantly affected (p-value = 0.124). A noticeable minimum threshold of approximately 375 ppm was observed among all species.
Discussion
Among these species examined, corals experienced health effects when exposed to hostile levels of pH, alkalinity, and calcium. However, it is important to note that P. decussata experienced no significant changes in any parameter, indicating that coral species have different responses in hostile environments. For instance P. decussata experienced a slightly higher Fv/Fm within the high calcium treatment compared to C. furcata and P. damicornis. All species reacted negatively to low calcium concentrations (300-350 ppm) with an apparent minimum threshold around 375 ppm. This threshold should be considered when maintaining aquacultures.
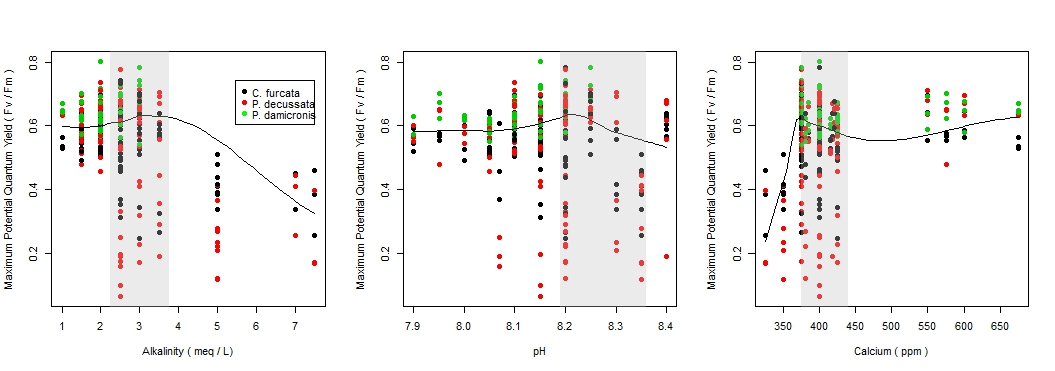
Figure 3. The changes of the maximum potential quantum yield with the changes of pH, alkalinity, and calcium by species. The shaded boxes represent the ranges for recommended for coral. The positive relationship of alkalinity and pH and the negative relationship of alkalinity and calcium should be considered when interpreting results.
Aquarist most often maintain elevated levels of pH, alkalinity, and calcium to promote coral health. In this study however, elevated conditions of these parameters resulted in a decline of photosynthetic efficiency (Fv/Fm), indicating an overall decrease in coral health. There was also a slight decrease in health as parameter values increased within the recommended ranges. This effect should be considered when dosing in a closed system as corals experienced optimal health within the lower values of the recommended ranges.
Ocean Acidification
While there were no significant changes observed in coral health between pH treatments, it is important to note that hydrochloric acid was used in place of carbon dioxide. While pH was altered, there was no net increase of carbonic acid, as projections indicate as a result of carbon dioxide sequestration. A decrease in pH is known to decrease the net calcification of hermatypic corals due to the reduction of alkalinity; however, photochemical efficiency showed no significant change during this study. This is important in that projected pH levels (i.e. 7.9) do not appear to significantly affect the photochemistry of zooxanthellae. This indicates the need for better understanding of the relationship between zooxanthellae and their coral host to accurately predict the changes in population dynamics that may result from ocean acidification.
Coral Aquaculture
As shown by Zartler (2012), individual coral species respond differently to changes in temperature within the recommended range for aquaculture. Similarly, this study shows that individual species respond differently to pH, alkalinity, and calcium. For instance, P. decussata and C. furcata are highly adaptive corals that have a small negative response in hostile water quality environments, and can be considered as ideal beginner corals. Whereas, P. damicornis has a smaller range of tolerance and would be more affected in the event of overdosing. This information can be used when determining the skill level required of an aquarist to maintain a specific species (easy/moderate/difficult), and should be considered when devising dosing procedures for aquaculture.
| Tank | Parameter | Parameter Goal | Parameter Mean | Additive |
|---|---|---|---|---|
| 1 | High pH | 8.7 | 8.28 | Limewater |
| 2 | Low pH | 7.7 | 8.07 | Hydrochloric acid |
| 3 | High Alkalinity | 5.0-8.0 meq/L | 4.9 meq/L | Seachem’s Alkalinity |
| 4 | Low Alkalinity | ~1.0 meq/L | 2.0 meq/L | Seachem’s Alkalinity |
| 5 | High Calcium | 500-800ppm | 530ppm | Seachem’s Calcium |
| 6 | Low Calcium | 250-300ppm | 386ppm | Seachem’s Calcium |
| 7 | Reference | Optimal | – | None |
Literature Cited
- Barnes, D. (1970). Coral Skeletons: An Explanation of Their Growth and Structure. Science. 170: 1305-1308.
- Brown, B. (1997).Coral Bleaching: causes and consequences. Coral Reefs. 16, S129-S138.
- Donowitz, R. (2002). Reefkeeping 102: Terms and Concepts to be Grasped. Advanced Aquarist. 1.
- Edinger, E., Jompa, J., Limmon, G.V., Widjatmoko, W., and Risk, M.J. (1998). Reef Degradation and Coral Biodiversity in Indonesia: Effects of Land-based Pollution, Destructive Fishing Practices, and Changes Over Time. Marine Pollution Bulletin. 36: 617-630.
- Freudenthal, H.D. (1962). Symbiodinium gen. nov. and Symbiodinium microadriaticum sp. nov., a zooxanthellae: taxonomy, life cycle, and morphology. Eukaryot Microbiology. 9:45-52.
- Heinz Walz GmbH. (2007). Junior-PAM chlorophyll fluorometer operator’s guide. 6: 43-46.
- Hilbertz, W. (1981) Mineral Accretion of Large Surface Structures, Building Components and Elements. United States Patent. 4,246,075.
- Holmes-Farley, R. (2005). What is that Precipitate in My Reef Aquarium?. Reef Central Magazine.
- Hughes, T.P., Baird, A.H., Bellwood, D.R., Card, M., Conolly, S.R., Folke, C., Grosberg, R., Jones, Hoegh-Guldberg, O., Jackson, J.B.C., Kleypas, J., Lough, J.M., Marshall, P., Nystrom, M., Palumbi, S.R., Pandolfi, J.M., Rosen, B., and Roughgarden, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science. 301: 929-933.
- Jones, R.J., Kildea, T., and Hoegh-Guldberg, O. (1999). PAM Chlorophyll Fluorometry: a new in situ technique for stress assessment in scleractinian corals, used to examine the effects of cyanide from cyanide fishing. Marine Pollution Bulletin. 38, 864-874.
- Kleypas, J. and Langdon, C. (2000). Overview of carbon dioxide induced changes in seawater chemistry. 9th International Coral Reef Symposium. 2: 1085-1090.
- Knowlton, N. and Rohwer, F. (2003). Multispecies Microbial Mutualisms on Coral Reefs: The Host as a Habitat. The American Naturalist. 162.
- Kramer, W.E., Schrameyer, V., Hill, R., Ralph, P.J., and Bischof, K. (2013). PSII activity and pigment dynamics of Symbiodinium in two Indo-Pacific corals exposed to short-term high-light stress. Marine Biology. 160: 563-577
- Langdon, C., Takahashi, T., Sweeney, C., Chipman, D., Goddard, J., Marubini, F., Aceves, H., Barnett, H., and Atkinson, M.J. (2000). Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochemical Cycles. 14, 639-654.
- Littman, R.A., van Oppen, J.H.M., and Willis, B.L. (2008). Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef). Journal of Experimental Marine Biology and Ecology. 364: 48-53.
- Marubini, F. and Atkinson, M.J. (1999). Effects of lowered pH and elevated nitrate on coral calcification. Marine Ecology Progress Series. 188: 117-121.
- Marubini, F. and Thake, B. (1999). Bicarbonate addition promotes coral growth. Limnology and Oceanography. 44: 716-720.
- McCowan, D.M., Pratchett, M.S., and Baird, A.H. (2012). Bleaching susceptibility and mortality among corals with differing growth forms. 12th International Coral Reef Symposium. 9A.
- Muscatine, L. and Porter, J. (1977). Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience. 27:454-460.
- Seachem Laboratories Incorporated. (2004). Marine Basic Test Kit Manual.
- Suwa, R., Hirose, M., and Hidaka, M. (2008). Seasonal fluctuation in zooxanthellar genotype composition and photophysiology in the corals Pavona divaricate and P.decussata. Marine Ecology Progress Series. 361: 129-137.
- Zartler, Z.K. (2012). Applying PAM fluorometry for the advancement of coral aquaculture. Advanced Aquarist.11: 28-34.



0 Comments