(1) Aquaculture and Fisheries Group, Department of Animal Sciences, Wageningen University, Wageningen University and Research centre, Wageningen, The Netherlands
(2) Coral Publications, Utrecht, The Netherlands
(3)Marine Animal Ecology Group, Department of Animal Sciences, Wageningen University, Wageningen University and Research centre, Wageningen, The Netherlands

Abstract
A long-standing debate amongst scientists and aquarists is whether corals can thrive on the photosynthetic excretions from their zooxanthellae, without any external organic food source. To provide more insight into whether corals can live such a “vegan lifestyle”, we conducted a basic, preliminary experiment. We cultured Seriatopora caliendrum colonies in virtually sterile water, providing light and inorganic nutrients only, as well as in a control aquarium where light and zooplankton were provided, for a period of 13 weeks. Although the corals which received only light and inorganic nutrients grew throughout the entire period, their growth rates declined substantially to nearly zero after thirteen weeks. In contrast, corals receiving light and zooplankton maintained normal growth rates. This preliminary experiment suggests scleractinian corals require an external organic food source in addition to light and inorganic nutrients to maintain normal growth, although more elaborate studies are warranted.
Introduction
It is common knowledge that reef-building corals live in symbiosis with dinoflagellates of the genus Symbiodinium, colloquially referred to as zooxanthellae (Muscatine et al. 1981; Muscatine 1990). These algae provide their coral host with various organic nutrients through photosynthesis (photoautotrophy), allowing corals to build vast coral reefs. In addition, corals are able to feed on organic matter (heterotrophy), including plankton, detritus and dissolved organic compounds, which can greatly enhance skeletal and tissue growth (reviewed by Houlbrèque and Ferrier-Pagès 2009; Ferrier-Pagès et al. 2011; Wijgerde 2013). Being able to utilize a combination of photoautotrophic and heterotrophic feeding, corals are sometimes referred to as mixotrophs.
Although it is known that heterotrophic feeding is beneficial to corals, a long-standing debate amongst scientists and aquarists is whether corals can thrive on merely the photosynthetic exudates from their zooxanthellae, without any external organic food source. To provide more insight into whether corals can live such a “vegan lifestyle”, thriving only on the exudates of their symbiotic algae, we conducted a basic, preliminary experiment. We cultured Seriatopora caliendrum colonies in virtually sterile water, providing light and inorganic nutrients (ammonium and phosphate) only, effectively creating a photoautotrophic feeding mode. As a control, we set up an additional aquarium with S. caliendrum colonies, which were provided with light and zooplankton. To evaluate coral health, we monitored weekly growth rates over a 13-week period.
Materials and Methods
Selected Species and Husbandry
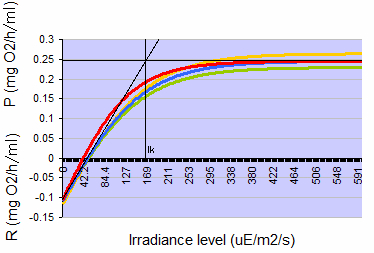
Figure 1: Typical photosynthesis-irradiance curves for Seriatopora caliendrum when acclimated to 150 µmol photons m-2 s-1, measured four times over a three month period on a single colony. Positive values indicate net photosynthesis (P), negative values indicate net respiration (R), both expressed as oxygen consumption in mg O2 per hour per ml coral volume. At 150 µmol photons m-2 s-1, photosynthesis rates are close to the Talling Index (Ik, onset of saturation), reached at ~160 µmol photons m-2 s-1 (µE photons m-2 s-1).
For this preliminary study, we used genetically identical colonies (i.e. clones) of the Indo-Pacific scleractinian coral Seriatopora caliendrum Ehrenberg 1834 (Pocilloporidae Gray 1842). An advantage of the Pocilloporidae is that their specific growth rates are rather stable, with exponential growth, also known as first order kinetics (Osinga et al. 2011, Leal et al. 2014). One large S. caliendrum parent colony was cut into smaller colonies, which were fixed onto PVC plates using two-component epoxy resin (Reef Construct, Aqua Medic GmbH, Bissendorf, Germany). Two colonies were used for photosynthesis measurements, and five were used for subsequent growth experiments. All colonies were allowed an acclimation period of three weeks before being used for the experiment. During this time, they were cultured in a 400 L aquarium at an irradiance level of 150 μmol photons m-2 s-1 as photosynthetically active radiation (~400-700 nm). The corals in the aquarium received zooplankton, i.e. 60 mL of concentrated Artemia nauplii suspension (approximately 3,000 to 5,000 nauplii mL-1) per day. A foam fractionator (MCE 600, Deltec GmbH, and Delmenhorst, Germany) was used to maintain water quality.
Analysis of energy budget: scope for growth
In order to pre-assess whether photosynthesis by itself would provide sufficient metabolic energy to sustain the corals’ requirements for growth and respiration, photosynthesis and dark respiration were determined by measuring oxygen evolution (cf. Schutter et al. 2008) on two colonies of S. caliendrum that had first been acclimated to a light intensity of 150 μmol photons m-2 s-1 for three weeks. The data were used to calculate under which light levels these corals had a positive phototrophic scope for growth, meaning that photosynthesis (in terms of oxygen and carbon produced) exceeds respiration of the coral during a 24-hour period. At a light level of 150 μmol photons m-2 s-1, photosynthesis rates were approximately 1.7 times higher than dark respiration rates (Figure 1), thereby resulting in a positive energy budget or scope for growth for this species. Although this calculation do not take carbon loss from mucus excretion into account, the amount of light used should be sufficient to grow this species well, at least in terms of organic carbon. Based upon this assessment, a light regime of 12 hours light and 12 hours darkness was used for the subsequent growth experiment, hereby maintaining an irradiance level of 150 μmol photons m-2 s-1.
Experimental Setup
After the three-week recovery period, the growth experiment was started. For the photoautotrophic treatment, small S. caliendrum colonies (N=2) were transferred to a 1.5 L incubation cell (Figure 2). Water temperature in the incubation chamber was set at 26.0 °C, by pumping fresh water through the cell’s water jacket from a container which was connected to a TC-15 thermostat (Teco, Ravenna, Italy). Illumination was provided by four T5 fluorescent lamps (Aquablue spezial, ATI, Hamm, Germany, 24W), at 150 μmol photons m-2 s-1 for 12 hours per day. A magnetic stirrer (IKA Werke GmbH & Co. KG, Staufen, Germany) provided water flow, at a rate of 5 to 10 cm s-1. Artificial seawater was produced with deionized water and Reef Crystals (Aquarium Systems, Sarrebourg, France). To supply inorganic nutrients, ammonium chloride and sodium hydrogen phosphate were added (NH4Cl, final concentration 0.6 μM or 0.01 mg NH4+ L-1, Na2HPO4, final concentration 0.05 μM or 0.005 mg PO43- L-1). This was constantly supplied to the cell by a peristaltic pump, which was connected to two jerry cans (10 L each). The jerry cans were kept in a refrigerator set at 5 °C to slow down bacterial growth in the seawater, which otherwise may have constituted a heterotrophic food source. To prevent the formation of pelagic bacteria and algae in the cell, caused by light and mucus excretion by the coral, a high water turnover was applied. Water replacement rate in the cell was approximately 2.8 liter per day, or about 100% every 13 hours. This also ensured nearly constant and natural ammonium and phosphate concentrations within the cell, allowing the zooxanthellae to grow. In addition, a small UV unit with 0.5 µm particulate filter (UV1, Pure Water Products, LLC, Denton, USA) was used to prevent growth of planktonic life, such as viruses, bacteria, fungi, algae and protozoa. The inside wall of the cell was wiped twice a week to prevent the growth of benthic algae, which otherwise may feed bacteria and corals via photosynthetic exudates. No organic feed was provided.
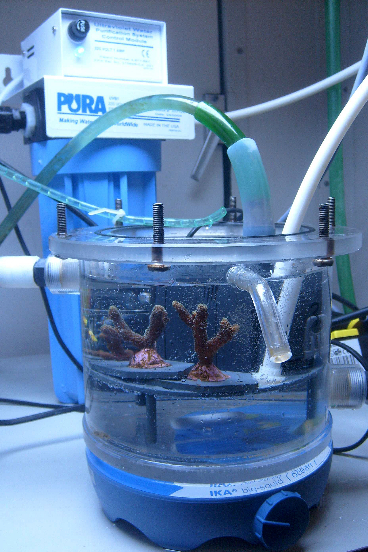
Figure 2: Experimental cell in which the corals were grown photoautotrophically. Water was changed constantly used a peristaltic pump, and a UV-unit prevented growth of microorganisms in the cell.
For the mixotrophic control treatment, S. caliendrum colonies (N=3) of similar size were cultured in the same 400 L holding aquarium as described above. Light was maintained at the same intensity as in the experimental cell, at 150 μmol photons m-2 s-1. These corals received 60 mL of concentrated Artemia nauplii suspension per day. Water parameters in this aquarium were maintained similar to that of the experimental cell (Table 1).
Temperature and salinity were measured with a TDS meter (WTW GmbH, Weilheim, Germany). Dissolved oxygen was measured with IntelliCAL™ LDO101 luminescent dissolved oxygen probes, connected to an HQ40d multimeter (Hach-Lange GmbH, Düsseldorf, Germany). Similarly, pH was measured using pH probes connected to an HQ40d multimeter (Hach-Lange GmbH, Düsseldorf, Germany). Alkalinity, calcium, magnesium, nitrate and phosphate levels were monitored using home test kits (Salifert BV, Duiven, The Netherlands).
Specific Growth Rates
To determine specific growth rates, buoyant mass of all five colonies was determined every Friday for 13 weeks, using the method of Davies (1989). Buoyant mass provides a good indication of skeletal mass increase, since coral tissue has nearly the same density as seawater and therefore hardly contributes to buoyant mass (Schutter et al. 2008). Measurements were done by suspending each colony, attached to a perforated PVC plate, on a hook. The hook was attached to an analytical balance (model 300, A&D, Tokyo, Japan) in a defined volume of seawater at constant depth. The seawater in the weighing chamber was maintained at 25 °C and a salinity of 35 g L-1. All buoyant weights were corrected for the mass of each PVC plate and epoxy resin. Specific growth rates were calculated with the following formula:
μ = (Wn-Wn-1)/Wn-1)*100
where μ is the growth (%) per week, and Wn and Wn-1 are the corrected buoyant weights in grams of each coral colony at the end and beginning of the week, respectively.
Results and Discussion
Corals from the photoautotrophic treatment exhibited an increasing growth trend in the first 4 weeks of the experiment (Figure 3). However, in week 5, a marked decrease in growth rate was observed. From week 7, growth rates clearly declined. In Week 13, growth rates had decreased to a marginal 0.1 and 0.3% for corals 4 and 5, respectively.
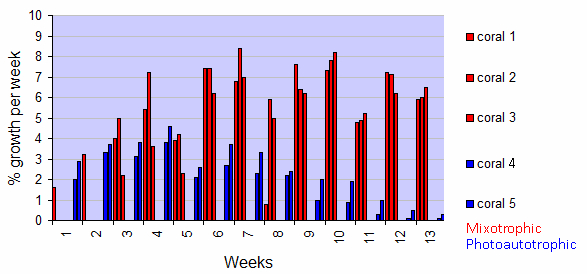
Figure 3: Growth of five Seriatopora caliendrum colonies during a 13-week experiment, with both light and inorganic nutrients (photoautotrophic treatment) or light and zooplankton (mixotrophic treatment). Values are individual growth rates expressed as % growth per week, of the photoautotrophic (blue) and mixotrophic (red) treatments.
In contrast, corals from the mixotrophic treatment show a different pattern. They showed a stronger increasing growth trend in the first 4 weeks of the experiment, but with a similar growth dip in week 5 (Figure 3). Beyond, their growth remained variable but high during the entire experimental period (save for week 8), with growth rates of 5.9%, 6.0%, and 6.5% for corals 1, 2 and 3, respectively, in week 13.
As sufficient light was provided (Figure 1), the most likely explanation for the observed decreasing growth rates of the photoautotrophic corals is malnourishment. A growing body of evidence suggests that scleractinian corals benefit from heterotrophic feeding, which could provide essential nutrients for tissue and organic matrix synthesis (reviewed by Houlbrèque and Ferrier-Pagès 2009; Ferrier-Pagès et al. 2011; Wijgerde 2013). The organic matrix is a proteinaceous framework which runs throughout the coral skeleton, and is essential to its formation (Allemand et al. 2004; Wijgerde 2014a). A key compound in this respect may be the amino acid aspartic acid (aspartate), a dominant component in organic matrices of coral skeletons and sclerites (Mitterer 1978), and which is also found in marine phytoplankton and zooplankton, including Artemia nauplii (Guisande et al. 1999). Without sufficient aspartic acid from an external source, Seriatopora caliendrum colonies may not be able to continue calcification, i.e. skeleton formation, at normal rates (Allemand et al. 1998, 2004). Although recent studies suggest both corals and zooxanthellae are able to synthesize aspartic acid (reviewed by Wijgerde 2014a), its biosynthesis may have to be complemented by external sources. This theory is plausible, as the morphology of scleractinian coral polyps strongly suggests they are highly adapted to prey capture (Yonge 1930); their cnidocyte-laden tentacles, coupled with mucus secretion, allow for effective capture and paralysis of nutritious prey (Wijgerde et al. 2011 and references therein). Indeed, we confirmed effective capture and uptake of Artemia nauplii by two Pocilloporids in the laboratory, including S. caliendrum (Wijgerde 2010a,b).
Although this preliminary experiment seems to have straightforward results, certain caveats may be raised. Table 1 lists several water parameters critical to coral growth during the experiment. It shows that various differences between both treatments occurred. Notably, water temperature in the experimental cell was lower due to cold seawater pumped from the refrigerator. In addition, oxygen levels in the experimental cell were lower, possibly due the lack of aeration. Finally, magnesium levels in both treatments were lower than natural. Despite these shortcomings, temperature, oxygen and magnesium levels remained within acceptable levels for coral growth (Wijgerde et al. 2012a,b; 2014b). Alkalinity levels were higher than natural in both treatments, especially in the experimental cell. However, these elevated values are known to have a positive effect on coral growth, including those of Pocilloporids (Hylkema et al. 2015). Finally, nutrients were undetectable using standard laboratory test kits, which was due to the low, natural levels present in the source seawater. As significant water changes were performed daily, with close to natural nutrient concentrations, a lack of inorganic nutrients is also unlikely to have occurred. This was substantiated by a healthy, pigmented appearance of the two corals in the experimental cell. Thus, the water parameters measured in the experimental cell are unlikely to have caused the observed growth decline in the photoautotrophic treatment.
| Parameter | Control aquarium (mean±s.d.) | Experimental cell (mean±s.d.) |
|---|---|---|
| Temperature (°C) | 26.9±0.55 | 24.5±1.7 |
| Salinity (g L-1) | 33.8±0.90 | 34.4±0.8 |
| Oxygen (mg L-1) | 7.88±0.77 | 6.60±0.92 |
| pH | 8.04±0.17 | 8.10±0.14 |
| Alkalinity (mEq L-1) | 3.06±0.97 | 4.46±0.21 |
| Calcium (mg L-1) | 454±70 | 438±29 |
| Magnesium (mg L-1) | 1133±57 | 1145±10 |
| Nitrate (mg L-1) | <0.2 | <0.2 |
| Phosphate (mg L-1) | <0.03 | <0.03 |
Besides water quality, it is possible that the UV unit produced damaging ozone and reactive oxygen species, which may have negatively affected corals from the photoautotrophic treatment. However, as we constantly changed the cell’s seawater, accumulation of such harmful substances is unlikely to have occurred.
A limitation of our preliminary study is that the control aquarium differs from the experimental cell in several respects, most notably in terms of size and shape. In addition, we only used one aquarium replicate for both treatments, preventing us from evaluating the reproducibility of the experiment. Finally, as we only used one species, and one genotype, we cannot at present confirm the generality of our observations. Therefore, we recommend conducting more elaborate, controlled experiments using multiple aquarium replicates, with various species and genotypes.
In conclusion, our preliminary results suggest that reef-building corals cannot thrive solely on the photosynthetic exudates of their symbiotic algae, and require an external organic food source such as zooplankton to maintain normal growth rates. Thus, scleractinian corals do not seem to be the ultimate “vegans”. This matches well with the morphology of coral polyps, which seem well-adapted to prey capture, as well as with previous observations on the beneficial effects of feeding on coral growth. Thus, the adage “fed corals are happy corals” seems appropriate still. For aquarists, there is no need for concern, as most aquaria receive plenty organic carbon in the form of various frozen and dry feeds.
Acknowledgements
We wish to thank Sietze Leenstra, Aart Hutten, Wian Nusselder, Sander Visser, Truus Wiegers-van der Wal and Ronald Booms for technical support.
References
- Allemand D, Ferrier-Pagès C, Furla P, Houlbrèque F, Puverel S, Reynaud S, Tambutté É, Tambutté S, Zoccola D (2004) Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C R Palevol 3:453-467
- Allemand D, Tambutté E, Girard JP, Jaubert J (1998) Organic matrix synthesis in the scleractinian coral Stylophora pistillata: role in biomineralization and potential target of the organotin tribulyltin. J Exp Biol 201:2001-2009
- Davies PS (1989) Short-term growth measurements of corals using an accurate buoyant weighing technique. Marine Biology 101:389-395
- Ferrier-Pagès C, Hoogenboom M, Houlbrèque F (2011) The role of plankton in coral trophodynamics, 215-229. In: Dubinsky Z, Stambler N (Eds), Coral reefs: an ecosystem in transition. Springer, Dordrecht, The Netherlands
- Guisande C, Maneiro I, Riveiro I (1999) Homeostasis in the essential amino acid composition of the marine copepod Euterpina acutifrons. Limnol Oceanogr 44:691-696
- Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev Camb Philos 84:1-17
- Hylkema A, Wijgerde T, Osinga R (2015) Decreased growth of Stylophora pistillata with nutrient-driven elevated zooxanthellae density is largely explained by DIC limitation. Advanced Aquarist 14(5)
- Leal MC, Ferrier-Pagès C, Petersen D, Osinga R (2014) Coral aquaculture: applying scientific knowledge to ex situ production. Reviews in Aquaculture 6:1-18
- Mitterer RM (1978) Amino Acid Composition and Metal Binding Capability of the Skeletal Protein of Corals. Bull Mar Sci 28:173-180
- Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals, 75-87. In: Dubinsky Z (Ed), Coral reefs: ecosystems of the world 25. Elsevier, Amsterdam, The Netherlands
- Muscatine L, McCloskey LR, Marian RE (1981) Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr 26:601-611
- Osinga R, Schutter M, Griffioen B, Wijffels RH, Verreth JAJ, Shafir S, Henard S, Taruffi M, Gili C, Lavorano S (2011) The biology and economics of coral growth. Mar Biotechnol 13:658-671
- Schutter M, van Velthoven B, Janse M, Osinga R, Janssen M, Wijffels R, Verreth J (2008) The effect of irradiance on long-term skeletal growth and net photosynthesis in Galaxea fascicularis under four light conditions. Journal of Experimental Marine Biology and Ecology 367:75-80
- Wijgerde T (2010a) Stylophora pistillata capturing and ingesting Artemia nauplii. YouTube video. https://www.youtube.com/watch?v=9dloJs9LMR4
- Wijgerde T (2010b) Seriatopora caliendrum capturing Artemia nauplii. YouTube video. https://www.youtube.com/watch?v=3vKwcjqwEK8
- Wijgerde T (2013) Coral Feeding: An Overview. Advanced Aquarist 12(12)
- Wijgerde T (2014a) Amino Acids and Corals: Sources, Roles and Supplementation. Advanced Aquarist 13(3)
- Wijgerde T, Diantari R, Lewaru MW, Verreth JAJ, Osinga R (2011) Extracoelenteric zooplankton feeding is a key mechanism of nutrient acquisition for the scleractinian coral Galaxea fascicularis. J Exp Biol 214:3351-3357
- Wijgerde T, Henkemans P, Osinga R (2012a) Effects of irradiance and light spectrum on growth of the scleractinian coral Galaxea fascicularis – Applicability of LEP and LED lighting to coral aquaculture. Aquaculture 344-349:188-193
- Wijgerde T, Jurriaans S, Hoofd M, Verreth JAJ, Osinga R (2012b) Oxygen and Heterotrophy Affect Calcification of the Scleractinian Coral Galaxea fascicularis. PLoS ONE 7(12): e52702. doi:10.1371/journal.pone.0052702
- Wijgerde T, Silva CIF, Scherders V, van Bleijswijk J, Osinga R (2014b) Coral calcification under daily oxygen saturation and pH dynamics reveals the important role of oxygen. Biology Open doi: 10.1242/bio.20147922
- Yonge CM (1930) Studies on the physiology of corals. I. Feeding mechanisms and food. Sci Rep Gt Barrier Reef Exped 1:13-57



0 Comments