A wide variety of organisms in reef tanks lay down calcium carbonate structures, including corals, mollusks, and algae. These structures provide a variety of functions, including protection and body support, and the process of calcification itself may increase photosynthetic efficiency. All reef aquarists are versed in the fact that such organisms remove calcium and carbonate from the water column in order to provide materials for calcification. Exactly how calcification takes place, however, is rarely considered. To a large extent the lack of attention to this detail probably relates to the fact that many of the chemical and biochemical details are simply not known. This article, the third in a series on calcium in reef tanks, will explore what is known and what isn’t about how calcification takes place.
In addition to the mechanistic details, this article will explore a variety of issues that relate directly to reefkeeping. These issues include the impact of changes in calcium, alkalinity, and pH on calcification. It will also briefly discuss how ions such as strontium get into calcium carbonate skeletons, and how phosphate inhibits calcification.

Figure 1. The site of calcification in a coral. At the bottom is the coral skeleton and at the top is the coelenteron of the coral. Calcium makes its way from the water column to the coelenteron, across the calicoblastic epithelium, and into the ECF where it is precipitated as calcium carbonate
Basics of Calcification
We’ll start by describing what most scientists agree on about how calcification takes place (whether it has been experimentally proven or not). The details can be different for different organisms, so the focus will be on corals, but calcifying algae are similar in many respects.1
In the case of corals, calcification takes place external to the organism. If one thinks of corals as tissue coating a calcium carbonate skeleton, then calcification takes place underneath the lowest layer of tissue (the calicoblastic epithelium, also called the basel epithelium) in a very thin water space called the extracytoplasmic calcifying fluid2 (ECF; Figure 1). The calicoblastic epithelium is attached to the crystal surface (the epitheca in many corals) with desmocytes, also called desmosomes3. These can be thought of as sheets of tissue running between the calicoblastic epithelium and the skeleton. The ECF is partially in contact with the surrounding water column. That is, the ions in this area can leak out into the water column, and ions there can leak in.2 Nevertheless, it is protected enough for calcification to be rapid there and slow to nonexistent to leak out in the bulk water.
Overall, corals must somehow get calcium from the water column to the ECF. They also must get carbonate to the same location. For calcium, the pathway is fairly clear, if not the exact transport mechanisms. Calcium travels from the water column, into the coelenteron (the gastric cavity of the coral), enters the calicoblastic epithelium, and then is transferred to the ECF. Additionally, it is generally agreed that somewhere along the line, active transport involving chemical energy is required, and that it is most likely involved in the transfer of calcium from the calicoblastic epithelium to the ECF.
The transport of carbonate is less clear, with scientists disagreeing on the details. Some suggest that bicarbonate enters the coelenteron from the water column and from metabolic processes in nearby cells. The bicarbonate combines with a proton to form CO2, and the CO2 freely diffuses across the calicoblastic epithelium to the ECF, where it is converted into carbonate. Other scientists believe that bicarbonate itself is actively pumped from the coelenteron across the calicoblastic epithelium to the ECF. Once in the ECF, it is converted into carbonate.
Once in the ECF, corals must still combine the calcium and carbonate to form aragonite (a particular crystal form of CaCO3). Corals cause growth of the calcium carbonate skeleton by raising the supersaturation of calcium carbonate to the point where precipitation is likely. In a previous article in this series, the factors that induce crystallization and those that inhibit it were described. In this context, corals do the following to initiate and control precipitation:
- They boost the calcium concentration by actively pumping calcium into the ECF.
- They may pump bicarbonate into the ECF as the source of carbonate.
- They may allow CO2 to freely diffuse into the ECF as the source of carbonate.
- They pump protons out of the ECF to convert CO2 and bicarbonate into carbonate.
- They maintain a fresh crystal face of aragonite as a seed for calcification.
- They may partially exclude magnesium, phosphate, and organics that inhibit crystal formation.
- They may help direct the crystallization with their own organic molecules, including proteins and polysaccharides, secreted into the ECF.
Conditions in the ECF
Unfortunately, the calcium concentration in the ECF is not known. Nor is the carbonate concentration. Nor, for that matter, is the pH, or even the alkalinity. In fact, is there any experimental information about it? To be honest, there isn’t much. Most of the information available involves theoretical predictions, such as McConneaughey and Whelan’s computation of the supersaturation that could result from a particular calcium and proton transporter that they believe exists (see details below).1 Apparently, the technical hurdles to measuring these values in the very thin ECF have proven too great.
One of the things that is known is the flux of calcium across the ECF. Values as high as 1.7 mol/cm2/h have been measured based on the overall calcification rate.2 One way to gauge the magnitude of this process is to compare it to seawater. Normal seawater is approximately 10 mM calcium. One cubic centimeter consequently contains 10 mmole of calcium. If the ECF had calcium at normal seawater concentration, the calcium would be totally depleted from approximately 1.7 mm of fluid above the skeleton in an hour. Of course, the ECF is not nearly this thick.
Unfortunately, the thickness of the ECF is also not well established, and likely not even constant.3 The calicoblastic epithelial cells themselves are about 1 micron thick, and the ECF area appears by microscopy to be even thinner. If we take a value of 1 micron for discussion sake, then the calcium within the ECF is totally depleted every 2 seconds. Consequently, while we do not know the calcium concentration (and hence the supersaturation), we do know that calcium is streaming across the ECF like water through a fire hose.

Figure 2. The site of calcification in a coral showing the proposed Ca++/2H+ transporter (green oval) that sends calcium from the calicoblastic epithelium into the ECF. The diffusion of CO2 from the coelenteron to the ECF and subsequent conversion into carbonate is also shown.
Calcium Transport from the Calicoblastic Epithelium to the ECF: Proton Antiport
If both calcium and a source of carbonate get into the ECF, it must come from the calicoblastic epithelium. How these materials get into the ECF is an area of debate. This first section will present the theory published by McConneaughey and Whelan1 (Figure 2).
Their proposal is that there is a calcium/proton antiporter that takes calcium from the interior of the calicoblastic epithelium and pumps it into the ECF. At the same time that each calcium ion enters the ECF, two protons are pumped back out of the ECF into the cell. Since both of these processes are transporting against a concentration gradient, it requires substantial energy. The energy in this case is provided by one adenosine triphosphate molecule (ATP) in the cell being broken down into adenosine diphosphate (ADP) and phosphate (a common driving mechanism for many transporters). These authors also point out that the actual transporter may be the related transporter where two calcium ions are transported for each four protons and one ATP molecule. If the actual transporter is one of these two, distinguishing between them is nontrivial since they have the same stoichiometry but different ATP requirements.
Relatives of these transporters are very common in other biological systems where calcium and protons are being exchanged, as on muscle cell membranes. In fact, their commonality may be the only reason that they have been suggested to be involved in calcification, and there appears little to no evidence that this transporter is actually present on the skeletal side of the calicoblastic epithelium.
One reason that active transport of calcium from the cell to the ECF is required is that the calcium concentration within the calicoblastic epithelium, as in nearly all cells, is much lower than it is in seawater. Typical concentrations of free calcium inside of cells are below 1 uM (though total concentrations can be mM when taking storage depots such as mitochondria into account) while in seawater the concentration is 10 mM. Transporting calcium ions against this gradient of 10,000x requires substantial chemical energy, and hence the need for ATP.1
We can, in fact, determine exactly how much of a gradient this transporter might theoretically pump against:
One ATP, used perfectly efficiently, is capable of driving calcium up a gradient of:
(1) E = 2.3RT[pCao – pCai]
Where E is the energy of hydrolysis of ATP, Cao is the calcium concentration in the ECF (o for Outside the cell), and Cai is the calcium concentration inside of the cell. Since E is about 50 kJ/mol, and RT at 25 °C is 2.48 kJ/mole, we can solve for the gradient:
(2) pCao – pCai = 8.8
That is, the calcium can be pumped up against a theoretical gradient of 6.3 x 108 . Consequently, if all that the transporter did was move calcium, and it did it with perfect efficiency, there is more than enough energy to drive the calcium from the cell to levels far above normal seawater in the ECF.
However, we also need to take into account the transfer of the protons. The energy required to pump them out depends on the pH inside the cell (pHi) and in the ECF (pHo):
(3) E = 2.3RT[2pHi – 2pHo]
where the “2” reflects the fact that 2 protons are being pumped. To gauge the effect, let’s assume that the pH in the cell is 7.0 and in the ECF is 9.0:
(4) pHi – pHo = -2
So
(5) E = 2.3RT[2pHi – 2pHo] = -22.8 kJ/mole
If we take the 50 kJ/mole from the original ATP hydrolysis and subtract out the energy required to pump the protons, we have:
(6) E = 2.3RT[pCao – pCai] = 50-22.8 kJ/mole = 27.2 kJ/mole
Solving we get
(7) pCao – pCai = 4.8
Consequently, we can see that there is enough energy available in one ATP molecule to pump calcium up a gradient of 104 and two protons up a pH gradient of 2 units. This gradient is adequate to cause supersaturation in the ECF (assuming that CO2 freely diffuses into the ECF or that H2CO3 otherwise gets there in adequate quantities).
Calcium Transport from the Calicoblastic Epithelium to the ECF: Other Mechanisms
There are a variety of other proposed mechanisms of calcium transport from the calicoblastic epithelium to the ECF.2 Tambutte et al4 have extensively studied the various calcium pools in one coral (Stylophora pistillata; Figure 3) and believe that this transport is dependent on a calcium-ATPase. This transporter, or a related Ca++-ATPase, has also been proposed by Isa et al5 and Ip et al6 . Unfortunately, there is little further characterization of these molecules in the context of a calcifying coral.
If the calcium-ATPase described by these authors is present in the bottom membrane of the calicoblastic epithelium as suggested, then it will have the same theoretical potential to boost calcium concentrations as the Ca++/ 2H+ transporter described above, without the proton transport (though there is some concern about how low the concentration in the calicoblastic cell can get before this transporter can no longer grab hold of calcium to begin with).2 Consequently, this Ca++-ATPase may be theoretically able to boost calcium to levels that induce supersaturation and would lead to formation of calcium carbonate.

Figure 3. A Stylophora pistillata coral similar to those used in many of the calcification studies described in this article. The picture was taken by Andy Hipkiss of a coral in his aquarium.
Carbonate Transport from the Calicoblastic Epithelium to the ECF: CO2
In McConneaughey and Whelan’s model, they propose that CO2 diffuses freely from the coelenteron to the ECF. CO2, in fact, does readily cross cell membranes when in its unhydrated form (CO2, not the hydrated form H2CO3). When the CO2 hits the high pH ECF, it is converted into carbonate, releasing two protons:
(8) H2CO3 ßà CO3– – + 2H+
The intrinsic simplicity of this model is that these can be the exact two protons pumped out by the Ca++/ 2H+ transporter (Figure 2). Consequently, the Ca++/ 2H+ transporter maintains a balance between calcium entering the ECF and protons leaving it.
The interconversion of CO2 and H2CO3 is a relatively slow process, taking seconds to minutes. Such a slow process would be problematic for a coral that needs to rapidly interconvert these two forms, and they consequently use an enzyme to speed up the process. Carbonic anhydrase is widely used by organisms including humans. In the context of corals, it is an important aspect of calcification.
When an inhibitor of carbonic anhydrase (e.g., acetazolamide or ethoxyzolamide) is added, calcification is decreased in a number of systems including Stylophora pistillata4 and a hydroid7 . This result does not say whether the required carbonic anhydrase is in the ECF or elsewhere (such as in the calicoblastic epithelium), but it is consistent with diffusion of CO2 into the ECF.
Carbonate Transport from the Calicoblastic Epithelium to the ECF: HCO3–
Of course, simplicity of a theory, as much as we might like it, does not necessarily mean reality. Other authors have provided evidence that active bicarbonate transport is important, not just CO2 diffusion. In fact, these two processes are not mutually exclusive: perhaps both contribute in different places, to different extents, or in different organisms.
Many authors have tried to distinguish between carbon coming from bicarbonate (or carbonate) in the water column and CO2 coming from metabolic processes. In most instances, authors conclude that both contribute to the pool of carbon going into the calcium carbonate skeleton. In Stylophora pistillata, 70-75% of the carbon going into calcification comes from metabolic processes, and 25-30% from bicarbonate in the water column8 . Still, the source of the carbon does not necessarily indicate how it gets to the site of calcification, as CO2 and bicarbonate are readily interconverted, especially in the presence of carbonic anhydrase.
Furla et al9 also claim that dissolved inorganic carbon (DIC; e.g., H2CO3/ HCO3–/CO3– –) is actively transported and that an inhibitor of anion transport (DIDS) halts the process:
“Seawater DIC is transferred from the external medium to the coral skeleton by two different pathways: from seawater to the coelenteron, the passive paracellular pathway is largely sufficient, while a DIDS-sensitive transcellular pathway appears to mediate the flux across calicoblastic cells.”
And also
“Irresp. of the source, an anion exchanger performs the secretion of DIC at the site of calcification.”
Still, both Furla et al and McConneaughey and Whelan may be partially correct: if bicarbonate is delivered to the ECF by an anion exchanger, there will rapidly become an excess of protons in the ECF, driving down the pH and inhibiting calcification. A removal mechanism involving Ca++/2H+ antiport solves this problem, though not with the perfectly balanced efficiency of the CO2 diffusion mechanism. Consequently, some active transport of protons from the ECF to the coelenteron must take place.2 Whether it takes place via McConneaughey and Whelan’s antiporter or some other mechanism remains to be established. In either case, how this large flux of protons across the calicoblastic epithelium takes place without disrupting the pH is unknown.2

Figure 4. The site of calcification in a coral showing the proposed Ca++ channel from the coelenteron into the calicoblastic epithelium (red oval) in addition to those features shown in Figure 3.
Entry of Calcium into the Calicoblastic Epithelium
The next question to answer is how the calcium enters the calicoblastic epithelium. Clearly, it does not just freely flow from the coelenteron because that space is largely in equilibrium with the external fluid (at least with respect to calcium in seawater; 10 mM calcium). If calcium did enter freely, the cell contents would also be at 10 mM calcium. While this might seem like an efficient process, cells do not survive well at 10 mM calcium, and need to keep the free concentration substantially lower as calcium plays a critical role in numerous cell processes and cannot be permitted to be unusually high.
Nevertheless, huge amounts of calcium are flowing across the calicoblastic epithelium, and somehow the inflow of calcium must be gated. In fact, there is a calcium channel that regulates the flow of calcium into the cell. This voltage dependent channel has been characterized, and a portion of it has even been cloned.10
This calcium channel does not pump calcium, but only allows it to flow in the direction of the concentration gradient (which is from the coelenteron into the calicoblastic epithelium; Figure 4). What it does do is open and close this calcium pathway in response to the voltage across the cell membrane. Such voltage dependant channels are very common in creatures of every type, but how they work on a molecular level is beyond the scope of this article. In short, however, the cell must change its surface electrical potential in response to its internal calcium concentration somehow, and this voltage change opens or closes the calcium channels.

Figure 5. A Galaxea sp. coral similar to those used in some of the calcification studies described in this article. This picture was taken by John Link of a coral his aquarium.
The evidence that this channel is important in calcification is substantial. Chemical inhibitors specific to these types of channels inhibit calcification in corals such as Galaxea fascicularis11 (Figure 5) and Stylophora pistillata.4 Immunohistochemistry has also been used to localize the cloned portion of the channel to the calicoblastic epithelium (as well as the oral ectoderm).
The Role of Organics
Organic molecules are known to play a substantial role in the formation of calcium carbonate in many organisms, including abalone shells12 and other mollusk shells13 . These materials can be proteins, glycoproteins, mucopolysaccharides, and phospholipids (and likely others that have not yet been identified). They help to induce the nucleation and growth of aragonite and are often referred to as the “organic matrix” because much of skeleton of corals is comprised of these organic materials.
In the case of corals, we have relatively little information about exactly what these organic materials are doing. The structures of some of these proteins contain an unusually large number of aspartic acid residues. These amino acids are capable of binding to calcium, but whether that is a critical function or not has not been established. Here is some speculation about what these organics might be doing with respect to calcification:
- They may help control the concentration of free calcium in the ECF, and thereby help control the rate of precipitation of CaCO3.
- They may control the location of crystal growth by binding free calcium and ferrying it to the location where the coral wants precipitation to take place.
- They may bind to the aragonite crystal face and thereby control the rate of precipitation.
- They may bind to the aragonite crystal face and thereby prevent precipitation in places where the coral does not want the skeleton to grow.
- They may bind to the aragonite crystal face and thereby inhibit binding of magnesium, phosphate, or other ions that are known to inhibit the growth of calcium carbonate crystals.
Regardless of the mechanisms involved, the need for these organics in calcification is easily verified. Allemand et al14 have studied the role of such materials in Stylophora pistillata. Interestingly, they find that inhibitors of protein synthesis reduce the rate of calcification considerably. For example, reducing protein synthesis by 60-85% reduced calcification by 50%. A similar result was found by inhibiting glycoprotein synthesis. These results did not come about because of reduced metabolism, but rather by specific effects of reduced protein and glycoprotein synthesis. The most important conclusion in their paper may be that the rate of skeletogenesis may be more limited by the rate of biosynthesis and exocytosis of organic matrix proteins rather than by calcium deposition.
Interestingly, the apparently large need for a particular amino acid (aspartic acid) to synthesize these proteins is satisfied by external sources, not by either the coral itself or the zooxanthellae. For this reason, it might be interesting to see what added aspartic acid does to calcification rates in reef tanks.
Implications for Reefkeeping: Calcium Concentration
Reefkeeping hobby lore has it that boosting the calcium concentration above natural levels of 410 ppm does little to enhance calcification in most corals. That idea is supported by experiments on Stylophora pistillata where calcification becomes limited by calcium at levels below natural levels, but is not increased above about 360 ppm.4 The relationship between external calcium concentration and calcification rate displays exactly the behavior to be expected if an active transport process were limiting the calcification rate, and that this transport process is saturated with calcium at concentrations above 360 ppm.
Using some of the information provided in previous sections, we can understand why this may be the case. Again, for Stylophora pistillata, as the calcium level is increased in an artificial seawater medium from 0 to 800 ppm, the calcium uptake by the coelenteron increases in a linear fashion.4 The uptake by most of the tissues other than the calicoblastic epithelium also increases in a linear fashion. There is no data specific to the calicoblastic epithelium, but the data show that calcification does not increase above 360 ppm calcium.
If the calcium is let into the calicoblastic epithelium by a calcium channel, then the influx of calcium is dependent on the concentration in the coelenteron, and the proportion of time that the calcium channels are open. Since the cells themselves control the gating of the calcium channels, they presumably can control their internal calcium levels at will UNLESS there is not enough calcium outside of the cells to go through the gate, cross the calicoblastic epithelium cells, and get to the active transporter that sends it into the ECF. Consequently, one interpretation is that at external calcium concentrations below 360 ppm, the calcium flux into the calicoblastic cells becomes the rate-limiting step in calcification.
There is a second interpretation that is also possible, however. In this scenario, calcium enters the calicoblastic epithelium through the gated channels, but is not controlled very well in the cell. As the calcium concentration in the coelenteron drops, the concentration inside of the cell drops (regardless of whether there is a large efflux or not), making it harder for the active transport to pump the calcium into the ECF, and thereby decreasing the rate of calcification.
The difference between these two scenarios is rather esoteric, and probably not of interest to most reefkeepers, but it is intellectually stimulating nevertheless. The difficulty in distinguishing these two scenarios comes about because the nature of the control of the calcium level in these cells is unknown. How exactly the large influx is regulated in relation to the large efflux is not understood and has apparently never been investigated.2 Consequently, we cannot yet know whether calcification drops primarily because the influx through the gates cannot keep up with the efflux rate when calcium concentrations in the coelenteron are low, or whether it drops primarily because the active transport of the calcium into the ECF cannot keep up when the calcium concentration in the calicoblastic cell is low.
Implications for Reefkeeping: pH
It is well known in the scientific literature, if not in the reefkeeping hobby, that calcification is slowed considerably as the pH is lowered below natural levels.15,16 This result is especially concerning and is a hot topic of research because of the decrease in the pH of the oceans as CO2 is added to the atmosphere. The predictions of reduced calcification in coral reefs in the future are substantial. Again, taking the previous sections as a backdrop, we can begin to understand why.
As the pH of the external fluid is lowered, it becomes harder and harder for cells to excrete the excess protons that come about from calcification. That is, they take in bicarbonate, strip off a proton, precipitate the carbonate into their skeleton and then have to do something with that proton. Many of those protons can be used to make CO2 out of bicarbonate, and may thereby boost the rate of photosynthesis.1 Still, not all of the protons may be used this way, and some will be excreted.
In the model of McConneaughey and Whelan,1 protons are pumped into the calicoblastic epithelium, and then allowed to somehow move down the concentration gradient from the lower pH cell interior (higher proton concentration) to the coelenteron (higher pH, lower proton concentration). If the seawater pH drops, the coelenteron pH will likely also drop since it is exchangeable with the external fluid. As the pH drops in the coelenteron, the efflux of protons from the calicoblastic epithelium cells will be slowed as that process is likely gradient dependent. Finally, as the pH drops inside of the calicoblastic epithelium because protons are not leaving as readily, it becomes harder and harder for the Ca++/2H+ transporter to pump protons out and calcium into the ECF.
In the model of Furla et al9 where bicarbonate is actively pumped into the ECF, protons must either be actively pumped away, or they must passively diffuse away (the latter probably is not efficient enough to work as the ECF could never then have a pH higher than the coelenteron, something that would mitigate against supersaturation and precipitation of aragonite). In either case, the process may not function as well at lower coelenteron pH as the proton gradient between the ECF and the coelenteron becomes smaller or more likely, bigger in the wrong direction. That is, protons will be less inclined to leave the ECF for the coelenteron. Consequently, the supersaturation of calcium carbonate, which is highly dependent on pH, will decrease, and hence calcification will also decrease.

Figure 6. A Porites sp. coral similar to those used in some of the calcification studies described in this article. The picture was taken by Joe Burger of a coral in his aquarium.
Implications for Reefkeeping: Alkalinity
Unlike the calcium concentration, it is widely believed that certain organisms calcify faster at higher alkalinity than in normal seawater. This result has also been demonstrated in the literature, where it has been shown that adding bicarbonate to seawater increases the rate of calcification in Porites porites17 Figure 6). In that case, a doubling of the bicarbonate concentration resulted in a doubling of the calcification rate.
Much, though not all, of the carbon source for calcification comes from external bicarbonate. As the alkalinity is reduced (at a given pH) the bicarbonate concentration (which comprises the bulk of the alkalinity in seawater) will also be reduced. Diffusion of bicarbonate or diffusion of CO2 from the coelenteron can apparently become rate limiting in many corals. In part this may be due to the fact that both photosynthesis and calcification are competing for bicarbonate, and the fact that the external bicarbonate concentration is not that large to begin with (when compared, for example, to the calcium concentration).
One Final Anomaly
As a final thought experiment, consider how materials other than calcium and carbonate get into the skeletons of corals. A variety of metals get into these structures, the most notable of these being strontium, but others include cadmium, manganese, lead, uranium and barium. How do they get there?
Apparently the relative concentrations of strontium (and other metals) and calcium in the ECF are similar to seawater2 based on skeletal incorporation rates in a large variety of coral species and locations. Once in the ECF, they get incorporated as impurities in the growing crystal in direct proportion to their concentration relative to calcium. The oddity is that these metals are able to get in and be precipitated in the same ratios in many different corals (though seemingly not all), and apparently in ratios suggesting that the relative concentrations are similar to bulk seawater. It has been claimed that the active transporter that sends calcium into the ECF can readily distinguish calcium from strontium, for example, and that strontium must enter in another fashion (e.g., in Galaxea fascicularis18 ). Why, if these metals enter in some other way, is the relative concentration so constant across many species of corals?
Consequently, it is a current topic of debate in the literature as to how these other metals become incorporated into aragonite skeletons. This topic is especially important, as the calcium to strontium incorporation rate in ancient coral skeletons has been widely discussed as a paleothermometer, with the incorporation rate varying in a known fashion with temperature. Understanding how the incorporation actually takes place would seem to be an important aspect of that hypothesis.
Another important issue has to do with the inhibition of calcification by phosphate and phosphate-containing organics. Phosphate is known to inhibit the precipitation of calcium carbonate from seawater.19, 20, 21 Phosphate also decreases calcification in corals, such as Pocillopora damicornis22 and entire patch reefs23 . This inhibition is likely related to the presence of phosphate in the ECF and on the growing crystal surface. Exactly how the phosphate gets in isn’t well understood. Nevertheless, the next time you are worried about phosphate levels in your tank, you can think of calcification inhibition in the ECF in addition to the driving of unwanted algae in your tank.
This inhibition of calcification takes place at concentrations frequently attained in reef tanks, and may begin at levels below those detectable by hobby test kits. For example, one research group found that long term enrichment of phosphate (2 mm; 0.19 ppm; maintained for 3 hours per day) on a natural patch reef on the Great Barrier Reef inhibited overall coral calcification by 43%.23 A second team found effects in several Acropora species at similar concentrations.24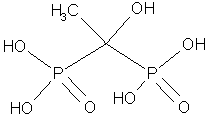
Organic phosphate and phosphonate inhibitors of calcification have also been studied and probably work by a similar mechanism. HEBP, a bisphosphonate that is shown below, causes a 36% inhibition of calcification in Stylophora pistillata at 10 mm, and stops it completely (99%) at 500 mm.25
The Story Continues….
There are a host of additional interesting topics related to calcification that have not been covered here. This article, for example, has not delved into the biological details of calcification, such as the nature of the tissues around the site of calcification. For those interested, some of these biological and mechanistic details are described by Barnes.3 This article has also not described the hypothesized relationship between calcification and photosynthesis, but it is an interesting one and worthy of understanding.1, 2 What this article has tried to accomplish is to provide a more detailed understanding of the chemical aspects of calcification taking place in your reef tank.
References
- Calcification generates protons for nutrient and bicarbonate uptake. McConnaughey, T. A.; Whelan, J. F. Marine Research, Biosphere 2 Center, Highway 77, PO Box 689, Oracle, USA. Earth-Sci. Rev. (1997), 42(1-2), 95-117.
- Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Gattuso, Jean-Pierre; Allemand, Denis; Frankignoulle, Michel. Observatoire Oceanologique, LOBEPM, UPRESA 7076 CNRS-UPMC, Villefranche-sur-mer, Fr. Am. Zool. (1999), 39(1), 160-183.
- The Structure and formation of growth-ridges in scleractinian coral skeletons. Barnes, D. J. Proc. Roy. Soc. Lond. B. (1972) 182, 331-350.
- A compartmental approach to the mechanism of calcification in hermatypic corals. Tambutte, E. Allemand, D. Mueller, E. and Jaubert, J. (1996) J. Exp. Biol. 199, 1029-1041.
- Evidence for the occurance of Ca2+-dependent adenosine triphosphotase in a hermatypic coral Acropora hebes (Dana). Sesoko Mar. Sci. Lad. Tech. Rep. (1980) 7:19-25.
- Some properties of calcium-activated adenosine triphosphatase from the hermatypic coral Galaxea fascicularis. Ip, Y. K.; Lim, A. L. L.; Lim, R. W. L. Dep. Zool., Natl. Univ. Singapore, Singapore, Singapore. Mar. Biol. (Berlin) (1991), 111(2), 191-7.
- Calcification in the planula and polyp of the hydroid Hydractinia symbiolongicarpus (Cnidaria, Hydrozoa). Rogers, Constance L.; Thomas, Mary Beth. Department of Biology, The University of North Carolina at Charlotte, Charlotte, NC, USA. J. Exp. Biol. (2001), 204(15), 2657-2666. CODEN: JEBIAM ISSN: 0022-0949.
- Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. Furla, Paola; Galgani, Isabelle; Durand, Isabelle; Allemand, Denis. Observatoire Oceanologique Europeen, Universite de Nice Sophia-Antipolis, Nice, Fr. J. Exp. Biol. (2000), 203(22), 3445-3457.
- Involvement of H+-ATPase and carbonic anhydrase in inorganic carbon uptake for endosymbiont photosynthesis. Furla, Paola; Allemand, Denis; Orsenigo, Maria-Novella. Observatoire Oceanologique Europeen, Centre Scientifique de Monaco, Monaco, Monaco. Am. J. Physiol. (2000), 278(4, Pt. 2), R870-R881.
- Cloning of a calcium channel a1 subunit from the reef-building coral, Stylophora pistillata. Zoccola, Didier; Tambutte, Eric; Senegas-Balas, Francoise; Michiels, Jean-Francois; Failla, Jean-Pierre; Jaubert, Jean; Allemand, Denis. Observatoire Oceanologique Europeen, Centre Scientifique de Monaco, Monaco, Monaco. Gene (1999), 227(2), 157-167.
- Calcification in hermatypic and ahermatypic corals. Marshall, A. T.. School Zool., LaTrobe Univ., Melbourne, Australia. Science (Washington, D. C.) (1996), 271(5249), 637-9.
- Direct Observation of the Transition from Calcite to Aragonite Growth as Induced by Abalone Shell Proteins Thompson, J. B., Paloczi, G. T., Kindt, J. H., Michenfelder, M., Smith, B. L., Stucky, G., Morse, D. E., Hansma, P. K. (2000). Biophys J 79: 3307-3312.
- Falini, G., Albeck, S. Weiner, S. and Addadi, L. (1996). Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271, 67-72.
- Organic matrix synthesis in the scleractinian coral Stylophora pistillata: role in biomineralization and potential target of the organotin tributyltin. Allemand, Denis; Tambutte, Eric; Girard, Jean-Pierre; Jaubert, Jean. J. Exp. Biol. (1998), 201(13), 2001-2009.
- Effects of lowered pH and elevated nitrate on coral calcification. Marubini, F.; Atkinson, M. J. Biosphere 2 Center, Columbia Univ., Oracle, AZ, USA. Mar. Ecol.: Prog. Ser. (1999), 188 117-121.
- Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Langdon, Chris; Takahashi, Taro; Sweeney, Colm; Chipman, Dave; Goddard, John; Marubini, Francesca; Aceves, Heather; Barnett, Heidi; Atkinson, Marlin J. Lamont-Doherty Earth Observatory of Columbia University, Palisades, NY, USA. Global Biogeochem. Cycles (2000), 14(2), 639-654.
- Bicarbonate addition promotes coral growth. Marubini, Francesca; Thake, Brenda. School of Biological Sciences, Queen Mary and Westfield College, London, UK. Limnol. Oceanogr. (1999), 44(3), 716-720.
- Are calcium and strontium transported by the same mechanism in the hermatypic coral Galaxea fascicularis? Ip, Y. K.; Lim, A. L. L. Dep. Zool., Natl. Univ. Singapore, Kent Ridge, Singapore. J. Exp. Biol. (1991), 159 507-13
- Kinetics of precipitation of calcium carbonate in seawater: role of phosphates and hydrodynamics of the environment. Pokrovsky, O. S.; Savenko, V. S. Mosk. Gos. Univ., Moscow, Russia. Okeanologiya (Moscow) (1993), 33(5), 681-6.
- Dissolution kinetics of calcium carbonate in sea water. V. Effects of natural inhibitors and the position of the chemical lysocline. Morse, John W. Dep. Oceanogr., Florida State Univ., Tallahassee, Fla., USA. Amer. J. Sci. (1974), 274(6), 638-47.
- Calcium carbonate retention in supersaturated sea water. Pytkowicz, R. M. Sch. Oceanogr., Oregon State Univ., Corvallis, Oreg., USA. Amer. J. Sci. (1973), 273(6), 515-22.
- Measurement of alizarin deposited by coral. Lamberts, Austin E. Dep. Zool., Univ. Hawaii, Honolulu, Hawaii, USA. Editor(s): Cameron, A. M.; Campbell, B. M.; Cribb, A. B. Proc. Int. Symp. Coral Reefs, 2nd (1974), Meeting Date 1973, 2 241-4.
- Effects of elevated nitrogen and phosphorus on coral reef growth. Kinsey, Donald W.; Davies, Peter J. Limnol. Oceanogr. (1979), 24(5), 935-40.
- ENCORE: the effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions Dennison, W.; Erdmann, M.; Harrison, P.; Hoegh-Guldberg, O.; Hutchings, P.; Jones, G. B.; Larkum, A. W. D.; O’Neil, J.; Steven, A.; Tentori, E.; Ward, S.; Williamson, J.; Yellowlees, D. Marine Pollution Bulletin (2001), 42(2), 91-120.
- The effects of HEBP, an inhibitor of mineral deposition, upon photosynthesis and calcification in the scleractinian coral, Stylophora pistillata. Yamashiro, Hideyuki. J. Exp. Mar. Biol. Ecol. (1995), Volume Date 1995, 191(1), 57-63.



0 Comments