In the last article, I discussed coral bleaching and its appearance in corals. In particular, I emphasized the variations in bleaching, and alluded to the fact that bleaching may, in fact, resemble other events that are distinctly not bleaching. In the next article, I will cover the final part of the series, white syndromes as they relate to coral diseases. In this article, I limit myself to those corals having white areas that are not caused by bleaching or disease.
I also mentioned that coral bleaching might be very hard to distinguish from tissue loss. Bleached corals have transparent tissue so that the skeleton is visible beneath it, or in some cases the tissue is very hard to see because it is greatly withdrawn. Here, various causes may evoke bleaching, tissue loss, or both.

Figure 1. The sharp adornments on the upper edge of coral septa can act like blades, cutting or piercing thin coral tissue. This Cynarina lacrymalis skeleton is very “toothed” and can easily puncture the thin inflated tissues in the living coral. Photo by Eric Borneman.
Mechanical Stress and Injury
Corals have very thin tissue that is easily damaged. It does not take much effort to cause injury or abrasion. Because coral tissue lies immediately superficial to skeleton, anything that breaks through two tissue layers (epidermis and gastroderm), themselves often composed of a single cell layer, will expose the white skeleton below. The simple act of moving a coral from place to place within an aquarium can result in finger pressure piercing tissue as the sharp skeletal elements break through the layers. Having just placed a few new corals in my own tank, I know that pressing the coral down onto an epoxy mount caused the septal adornments to pierce through the tissue of an_ Echinophyllia_ species. Similarly, corals that are overturned or broken in the wild, and corals tumbling from their placement in the aquarium, can sustain tissue injury that causes the skeleton to become exposed.

Figure 2. This Siderastrea siderea was overturned so that the white area was upside down on the sand. The discolored area adjacent to the white area is a common sign in stressed tissue. Photo by Eric Borneman.
Tearing or piercing is not, however, the only way injury can result in white areas on a coral skeleton. Corals are very susceptible to abrasion by sand, and to local bleaching or tissue loss by sedimentation. On the reef, shallow waters are subject to disturbances that can abrade tissue during periods of heavy wave action, especially when the sediments consist of sands. In the aquarium, this happens more frequently when a powerhead falls from its mounts onto the bottom of the tank. Powerheads and pumps can pose another hazard if dislodged since the force of water itself can blast tissue off corals. As an aside, some research utilizes a variation of this water blasting when coral tissue must be collected for study: Water Piks and airbrushes are used to remove coral tissue from the skeleton.
Another deleterious result of sediments is smothering. Fine particulate and organic flocculent material (detritus, silts, etc.) is easily borne into the water column where it can settle on coral surfaces. The mucus covering of corals, while generally helpful in removing such debris, is also a sort of sticky trap for such materials. While corals feed on organic particulates, excess can be
problematic for several reasons. First, accumulations of particulate matter prevent light from reaching the coral surface in the local area of settlement. This can result in bleaching. Second, particulate material can smother the tissue, resulting in local hypoxia (a deficiency of oxygen reaching the tissues) or anoxia (hypoxia that causes damage). A great deal of partial mortality, and even total mortality, in corals both on the reef and in aquariums occurs from sedimentation damage of this sort. When this happens, both bleaching and tissue loss can be the result. A final problem with smothering is that the often organically rich material is enriched with microorganisms, including bacteria, ciliates, cyanobacteria, fungi, and other flora and fauna that can directly or indirectly cause bleaching and/or tissue loss. Some of these components may even be primary pathogens, although the aspect of pathogenicity will be covered in the next article.

Figure 3. This Acropora palmata fragment shows three reasons for white areas. First, the growing margins are white, and this is normal as they are growing rapdily and do not yet have zooxanthellae. Second, a wire is visible that was used to attache these fragments in a reconstruction effort. The wire has caused whitened areas where the coral is growing over it. Finally, patchy white areas are seen on the front fragment, and these were caused by a broken wire abrading the surface. Photo by Eric Borneman.

Figure 4. The spots in the center of the coral are areas caused by sediment deposits. These are older, but sediments deposited on the coral surface will first appear white until they are grown over by other flora or fauna. Photo by Eric Borneman.

Figure 32. This coral, along with the entire reef, is being covered by sediments from coastal development. Such deposits endanger the tissue of living corals. Photo by Eric Borneman.

Figure 28. I was perplexed at this spotty white area on a Montastraea faveolata. It was not until examining the photo that I caught the perpetrator. Look closely nearby for a small face. Note how the upper surface tissue is gone, but the polyps beneath “mouth’s reach” remain in their corallite unscathed. Photo by Eric Borneman.
Predation and Competition
Probably the most common source of white corals in aquariums and in the wild results from the action of other coral reef plants and animals. All too often, corals show signs that are easily confused with bleaching or disease. Mistaken identifications are equally as common among aquarists as they are among trained scientists. At least two cases of predation have been officially mistaken as a “new” coral disease, with many more descriptions that are mislabeled as existing coral disease. When I mentioned that it was often difficult to tell the difference between conditions when corals have white areas, I was not exaggerating!
Rapid Wasting Disease was first mentioned on the mailing list serve for NOAA’s “Coral Health and Monitoring Program.” Soon thereafter, an article appeared in the newsletter for the International Society for Reef Studies, Reef Encounter (Cervino et al. 1997). The description of Rapid Wasting Disease was primarily for massive corals in the Caribbean showing signs of tissue loss, a sharp white band delineating healthy tissue from the disease band line, and the erosion and dissolution of the coral skeleton. Preliminary results indicated the presence of fungal hyphae and these were suspected of being the causative agent pending further study. Rapid Wasting Disease was discussed at length and reported from areas throughout the Caribbean, and even into the Pacific. It was on the verge of being deemed an epizootic. However, some researchers were suspicious that this was not a disease at all, but the grazing behavior of parrotfish. Andy and Robin Bruckner conclusively put Rapid Wasting Disease into the “false alarm category” with several papers that showed this condition to result from unique biting behaviors of parrotfish (Bruckner and Bruckner, 1998a, 1998b, 1998c, in press). Spot, focused, and repeat biting behavior (where the fish repeatedly bites the same spot on a coral, even after swimming away to return to the same spot later), is an interesting behavior with few explanations – worthy of its own study. It is not, however, a coral disease (Bruckner et al., 2000). The fungal hyphae are believed to be normal endolithic fungi or perhaps even those growing on parrotfish beaks.
Figures 5, 6, and 7. The stoplight parrotfish, Sparisoma viride, and others, participates in various biting behaviors that produce signs on a coral that were incorrectly thought to be a new disease called Rapid Wasting Disease.
This is not the only group of fish that bite corals, either. Many, including, damselfish, butterflyfish, triggerfish, pufferfish, angelfish, and even some gobies, nip or graze coral tissue or individual polyps. In such cases, spotty areas that may show partial or complete tissue loss are seen. Rarely are these bites as deep as they are with parrotfish, and small nips may not even break the tissue. However, local stress can result in bleaching of the area. Furthermore, there can be a continuum of pale or white areas as new bites are made and old bites heal. Therefore, the nature of these areas may not be easily recognizable.
Figures 8-12. Many fish may leave bite marks on coral that can be deceptive. These corals from both the Caribbean and the Pacific have characteristic patchy white marks that result from bite marks in various stages of healing.
Ridge Mortality Disease is another “infamous” false alarm (Abbot 1979, Bruckner, pers. comm.). Massive Caribbean corals such as Diploria spp. and Colpophyllia natans were reported to be losing tissue with a distinct pattern along their upper ridges. As with Rapid Wasting Disease, the initial description was soon followed by a slew of reports – another impending epizootic was at hand. I suppose the coral disease community has a right to be a bit paranoid considering the extent of real “new” diseases. It was noticed that the tissue loss along the ridges of affected corals shared two things: a proliferation of filamentous algae over time, and the presence of damselfish around the colony. Debbie Santavy, a coral disease researcher in Pensacola, Florida, admits that many cases re certainly attributable to damselfish activity, but she also feels that there is legitimate ridge mortality disease exclusive of damsels nipping and killing coral tissue to grow algal turfs as a food source (Santavy, pers. comm.).
Figures 13, 14, 36. These patchy white blotches are caused by damselfish. However, it may closely resemble a beginning white band disease or fireworm predation. Note the green algae on some of the branches; these farmed filamentous algae patches are why the fish nip the coral tissue.
Figure 15, 39. While no damselfish are visible, the algae on the older nipped skeleton almost assures one that they are nearby. This pattern of nipping the upper margins of the meanders was called Ridge Mortality Disease, although this is not a disease at all.
Many other organisms prey on corals or can induce signs of disease or bleaching by producing whitened areas on corals (Table 1). Among the more common denizen diners of coral are various corallivorous gastropods. The Caribbean fireworm, Hermadice carunculata, grazes many Stony Corals, and may even eat gorgonians and sponges. Large fireworms can swallow an entire branch of Acropora cervicornis. Grazing trails are frequently present, but not always. Its important to remember that Hermadice is the only polychaete known to eat coral (though many bore into skeleton), and unless live rock from the area is in the aquarium or the worm was introduced some other way, coral tissue loss due to errant polychaetes is probably not happening.
| Group | Mode | Common Aquarium Representatives |
|---|---|---|
| Algae | C | Caulerpa, Dictyota, Laurencia, Halimeda |
| Sponges | C, P | many possible |
| Soft Corals | C | See Aquarium Corals (Borneman 2001) |
| Stony Corals | P, C | See Aquarium Corals (Borneman 2001) |
| Tunicates | C | usually none |
| Bryozoans | C, P | usually none |
| Hydroids | C, P | many, unidentified |
| Anemones | P | Aiptasia, Condylactus, clownfish hosting anemones, others |
Figures 16, 17. While many think Hermadice carunculata only feeds on stony corals, I witnessed them feeding on sponges and gorgonians, as well. Fortunately, these worms are uncommon in aquaria and they are only found in the tropical western Atlantic and Caribbean.
Figure 18, 19. While seemingly impossible, Hermadice is capable of swallowing entire branches of Acropora cervicornis. These white areas devoid of tissue are often mistaken as a disease. Figure 18 photo by Dr. Andrew Bruckner.
Other common coral predators include the nudibranchs and corallivorous gastropods, most of which are probably specialists one or a few types of coral, at most. Therefore, the presence of white corals from many varied genera in the aquarium are probably not attributable to nudibranchs. Sea stars can feed on corals, and there is some amount of anecdotal evidence that the small white stars that reproduce in aquariums ( Asterina sp.) may consume some amount of coral tissue. Some may be opportunistic scavengers, others may avoid corals entirely. There are several highly corallivorous stars, the most infamous being Acanthaster planci, the crown-of-thorns starfish. These animals can consume entire table corals at a time, with thousands or even millions moving across and consuming corals on reefs when they occur in plagues. Interestingly, the primary predators of these stars are whelks, giant gastropods whose numbers have been significantly reduced over the past century for the curio trade. Also, effective defenders of corals are commensal crabs of the genera Trapezius and Tetralis. These tiny crabs inhabiting branching corals prevent sea star attack by either nipping off the “thorns” of Acanthaster, or snipping off their tube feet (Pratchett , Vyotopil and Parks 2000). Fortunately, Acanthaster and other large stars like Culcita sp. are not found in the aquarium trade and will not be a problem for aquarists. Crabs and shrimp may also dine on coral tissue, as will some urchins. Most urchin grazing is likely incidental and none are exclusively corallivorous. Many other organisms may bore into corals, through living tissue, and reside there. Generally, this does not harm the coral, but local areas of whitening near the bore hole may be present. A list of some known coral predators and borers is given in Appendix 1.

Figure 21. Barnacles and worms ( Spirobranchus gigantea pictured here) are common boring organisms in living corals. Many others exist, but they usually do little harm to the colony as a whole. Note the local whitening of coral tissue near the barnacle. Photo by Eric Borneman.

Figure 22, 23. These small nudibranchs cause the bleaching-like appearance of Montipora sp. They seem to be almost specific for Montipora predation. Photos: Tracy Gray

Figure 37. This gorgonian eating snail, Cyphoma gibbosum, is very beautiful. While not preying on stony corals, it is a significant consumer of seafans and other gorgonians, leaving exposed axial skeleton with no tissue in its wake. Photo by Eric Borneman.

Figure 38. A Drupella sp. snail is a common corallivore of Pacific stony corals. Photo by Eric Borneman.
Competition is another means by which corals may become whitened by bleaching and/or tissue loss. This includes direct physical competition and competition at a distance, usually by means of chemical toxins produced by various organisms. These toxins and noxins are also known as secondary metabolites or allelopathic substances. Aquarium Corals (Borneman 2001) covers this subject in some depth, but virtually all groups of benthic invertebrates and plants contain members that may produce compounds that can result in “white” corals through bleaching and/or tissue loss. A list of some of these is found in Table 1, with notable aquarium representatives highlighted. More direct competition occurs when corals use cnidocyst-laden tentacles and mesenteries, mesenterial extrusion, sweeper tentacles and other means to injure nearby and competing species. I use corals as an example, but there are many organisms with various direct and indirect competitive methods that can produce these effects. Sponges, tunicates, hydroids, algae and virtually all benthic life must somehow compete and may emerge completely or partially victorious, leaving their effects on the coral.

Figure 24. The Diploria strigosa in front is in direct competition with the Montastraea faveolata behind it. The Diploria appears to be the stronger competitor, and the tentacles reaching the nearby Montastraea tissue have created a white zone that appears bleached and may soon show tissue loss. This type of marginal effect is also where disease lines tend to form, and so the two could be easily confused. Photo by Eric Borneman.
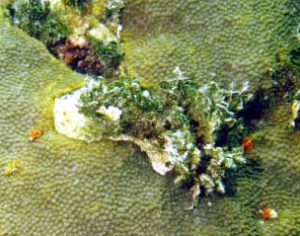
Figure 25. Algae can also be strong competitors. A bare zone exists around this Halimeda sp. Ordinarily, one would assume that sediments or other damage created a dead spot on the coral colony, onto which Halimeda merely settled and grew. However, the white area indicates ongoing effects, and is almost certainly due to the allelopathic effects of metabolites produced by Halimeda. This algae, while having defenses, is much less toxic than many. Photo: Deborah Lang

Figure 26. Sponges can certainly cause tissue loss or bleaching from their production of secondary metabolites. Here, only minor local effects are seen as the sponge tissue contacts coral tissue. Photo by James Wiseman.

Figure 34. Sweeper tentacles on a Euphyllia ancora are structures used by many corals to inflict damage on competing colonies. Photo by Eric Borneman.

Figure 35. Mesenterial filaments on a Hydnophora rigida will, upon retracting, have killed the entire contact region on the Stylophora pistillata. These structures can deploy and work very quickly, leaving dead tissue in their wake and an exposed white skeleton. Photo by Eric Borneman.
Recession, Starvation, and Senescence
A “catch all” phrase used to describe the progressive and usually slow loss of tissue is recession. Generally, receding tissue on corals show poor polyp extension and perhaps abnormal coloration (pale or otherwise), along with an area of bare corallites where polyps have died. This type of loss is usually slow enough that white areas are not seen because algae and other organisms colonize the skeleton at a rate that matches the tissue loss. Starvation is probably involved in many cases and certainly direct or intentional starvation can produce the same signs. Areas of recession tend to be at the older areas of a colony; near the base, within the center of foliose plates, etc. The reason recession often begins in these areas may be due to several factors: First, corals infill their skeletons as they grow, and older areas of a colony may not maintain good contact with other younger polyps near the growing margins. As such, they may become isolated and not be able to communicate with other polyps through the gastrovascular canals, losing a food source and perhaps other advantages to a colonial lifestyle. Second, these regions tend to have reduced water flow and light capture area, along with being protected from potential prey capture opportunities. As such, respiration and photosynthesis can be dramatically reduced and result in the local weakening or death of those polyps. Third, coral polyps are now known to show senescence. In simpler, if not somewhat incorrect, terminology, they are dying of old age. Any of these reasons can potentially cause areas of skeleton to be exposed, resulting in a patchy or even band-like white area that may be mistaken for any number of other problems. It bears repeating that it is very difficult to know the true reasons for these similar looking manifestations.

Figure 33. This Acropora has been hit by cyanide fishermen to collect small aquarium fish living in the branches. Toxic chemicals can bleach of kill coral tissue, leaving the characteristic white affected areas. Photo by Eric Borneman.
Others
There are many other things that can cause a white appearance in corals. Some of these were covered as agents of bleaching in the last article. However, some may also cause tissue loss in addition to, or in place of, simple loss of zooxanthellae. For example, chemicals, heat, radiation, and others can cause the direct death of coral tissue inclusive or exclusive of the loss of symbiotic algae. Of particular note to aquarists are accidental introductions (and from tragic stories I have heard, sometimes intentional ones by angry store employees, customers, and ex-relationship members) of things like bleach, pesticides, paints, and other aerosols. It should, therefore, be little wonder that the list of potential agents of tissue loss that result in white areas on corals is almost up to the imagination. There are only so many ways for a coral to die, and unfortunately the signs of death are almost invariably areas of visible or exposed white skeleton beneath, or lacking, healthy, pigmented, overlying tissue.
In the next article, I will describe some coral diseases that are collectively known as “white syndromes.” All too often, aquarists seeing signs that may resemble these diseases jump to the conclusion that a disease is, in fact, present. I hope this article, and the previous one, will help readers to assess each case carefully and with good observations. There are so many potential reasons for a coral to present visible white areas, and only by determining what the problem actually is, and also what it isn’t, can steps occur to minimize further loss and perhaps aid recovery
References
- Abbott, R.E. 1979. “Ecological processes affecting the reef coral populations at the East Flower Garden Bank, northwest Gulf of Mexico.” Ph. D. Dissertation. Texas A&M Univ., College Station, TX 154 pp.
- Borneman, Eric H. 2001. Aquarium Corals. Microcosm, T.F.H., neptune City. 464 pp.
- Bruckner, A.W. and R.J. Bruckner_. In press_. “Coral predation by Sparisoma viride and lack of relationship with coral disease.” Proc 9th Intern. Coral Reef Symp
- Bruckner_, A.W. , R.J. Bruckner and P. Sollins. 2000. “Parrotfish predation on live coral: “spot biting” and “focused biting”.” Coral Reefs_. 19:50.
- Bruckner, A.W. and R.J. Bruckner. 1998a. “Destruction of coral by Sparisoma viride.” Coral Reefs. 17:350.
- Bruckner, A.W. and R.J. Bruckner. 1998b. “Rapid wasting disease: pathogen or predator.” Science. 279. 2023-2025.
- Bruckner_, A.W. and R.J. Bruckner. 23 July, 1998c. “_Rapid-Wasting “Disease”: Coral predation by stoplight parrotfish.” Reef Encounters. pp.18-22.
- Cervino, J., T.Goreau, G. Smith, K. DeMeyer, I. Nagelkerken and R. Hayes 1997. “Fast spreading new Caribbean coral disease.” Reef Encounter 22:16-18.
- Pratchett, M., Vytopil, E., and Parks. P. 2000. “Coral crabs influence the feeding patterns of crown-of-thorns starfish.” Coral Reefs 19: 36.






















0 Comments