For one reason or another, there is no single perfect food organism for the culture of marine larval fish. Each species of fish has its own constellation of ecological and feeding requirements during its larval stage and is adapted for best survival in inshore or offshore waters with a particular combination of dominant ecological, physical, and chemical factors. It’s absolutely amazing, and a testimony to the resiliency, vigor, and adaptability of these tiny marine creatures that we can culture any of them in little glass boxes so far removed from the expanses of the open sea. Of course the intelligence and determination of the human species has a lot to do with it also.
In this months offering, Martin and I assist in feeding those finicky fry by providing two additional microfoods; one large -mysid juveniles and one small invertebrate veligers. These microfoods are supplements that one can add to your menu of assorted size food items to feed difficult fry. Both microfoods are on the advanced side of culturing, but if you can make it to the end of the column and you’re not lost, you should have sufficient background and experience to easily culture these food items!
Possum Shrimp (Mysis sp.)
Background: Mysids are small shrimp-like crustaceans with a heavy carapace covering their thorax and can grow to 1 cm in length (Reitsema & Neff 1980) [Fig1]. Adapted to life in estuaries, these tough, hardy crustaceans can withstand a wide range of salinities and temperatures. Mysids inhabit estuarine waters from Florida, USA, to the East Coast of Mexico (Bowman 1964). According to Price (1976), Mysidopsis almyra is the dominant mysid species in the estuaries surrounding Galveston Island, Texas, USA, comprising 82% of the mysids collected. Mysids (primarily Mysidopsis almyra or Mysidopsis bahia ) have been used extensively as indicator species in water toxicity tests (Miller 1990) for many years and are commonly reared or cultured in the laboratory (Reitsema & Neff 1980). Before the advent of lab culturing, Mysids were routinely collected via dip netting (McKenney 1996). Mysidopsis species are omnivorous and cannibalistic, feeding on diatoms and small crustaceans such as copepods (Mauchline 1980).
Life cycle
These crustaceans are commonly called possum shrimp because the females carry their developing young in a bulging pouch or marsupium formed by at the base of their legs. Females can carry broods of up 30 fry in their pouches, although 6 or 7 is the normal brood size. The young mysids are not released until they are well-developed juveniles. Each fry is approximately 4-to-5 times bigger than newly-hatched Artemia nauplii (baby brine shrimp). Females produce young continuously, refilling their pouch with eggs as soon as their latest brood is released. The juvenile Mysids will reach their adult size of 1 inch (1.25 cm) in about 3 weeks, creating a new generation every 30 days. Male mysids are slightly larger than female mysids and are readily identifiable by their conspicuous absence of the white brood pouch. Laboratory strains of tank-raised Mysids are available that have been selected for resistance to disease and are pre-adapted to aquarium life (see additional reading and online suppliers).
Husbandry
Despite their widespread use as pollution bio-assay organisms, Mysids are remarkably undemanding in terms of water quality (as long as the values remain within a reasonable range). According to Hemdal, no unusual mortality was noticed in tanks even when the ammonia concentration approached 1 ppm. Mysid density in a culture tank affects reproduction. According to Lussier et al. (1988), a culture that is overcrowded will cease reproduction, resulting in high proportion of females with empty brood sacs. The above authors indicated that the optimal densities were 15 adult mysids/ L in a flow-through system and 10 adult mysids/ L in a static culture [Fig 2]. The ability to culture mysids in large numbers at low cost enables the successful aquaculturist to raise several unique marine species, including leafy sea dragons and cephalopods (Hanlon, Turk & Lee 1991).
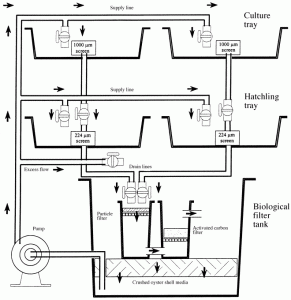
Fig 2. Diagram of a static mysid culture system. The two top tanks (culture trays) were used for holding broodstock, the two middle tanks (hatchling trays) were used for the hatchlings, and the bottom tank was the biological filter tank, containing a particle filter, activated carbon filter and a submerged, oyster shell biological filter. Water drained through the screened cores in the culture and hatchling trays into the biological filter tank. It was then drawn from below the crushed oyster shell media, and pumped back into the culture and hatchling trays through the supply lines.
Average water quality for mysid culture tanks (according to Hemdal):
- Temperature = 75°F
- Salinity = 20 – 22ppt
- pH = 8.2
- Light = 75 foot-candles
- Ammonia = 0.1 mg/l
- Nitrite = 0.01 mg/l
Enrichment Of Mysids
Enrichment has been shown to be an effective way to ensure nutritionally sufficient Mysid juveniles. The easiest way to enrich Mysids is to feed them fortified Artemia nauplii (Kuhn et al. 1991). Experiments in static (closed) systems indicate that enriched Artemia nauplii are the best food item for mysids (Domingues et al. 1998). Therefore, it is highly recommend to enrich both adult and hatchling mysids with Artemia nauplii fortified with marine fatty acids (such as phytoplankton-enriched or Selco® enrichment) for 12 h prior to feeding (see Breeder’s Net column). As a reminder, Artemia cysts are hatched for 24 h under intense fluorescent light at temperatures of 28 °C with salinity between 18 and 22, and are then collected on a 53um mesh screen. Enrichment of the newly hatched Artemia nauplii is accomplished by soaking the animals in a solution of Selco® and seawater at 0.25 g Selco® /L sea water for 12 h.
Culturing of Mysids
The following culture instructions are based on a method used by Lewis (Lewis, 2000) to raise Mysids on a commercial basis at Aquatic Indicators (see online suppliers list). It should be stressed that culturing Mysids is a fairly labor-intensive project; however mysid culture can be accomplished by anyone who is willing to put in the time and effort.

Fig 3 Mysid mass culture setup. 30 gallon tanks line this Laboratory culture room where many mysids and artemia are raised.
First, utilizing a 20-gallon tall or larger all-glass aquarium, add a standard undergravel filters at either end, but leave the center of the tank bare (no U.G.s) to facilitate collecting the Mysids. As an example, if you’re using a 30-gallon culture tank, install U.G. filter plates designed for a 10-gallon aquarium at both ends of the culture tank, but leave bare glass at the bottom in between the 2 filter plates. Adjust the specific gravity to about 1.022 and set the temperature at 75-78F.
Once the tank has cycled and the biofiltration is established, introduce 20-30 adult Mysids to get the culture started. Establish a photoperiod of 16 hours of light and 8 hours of darkness (Mysids mate at night), and perform 15-20% water changes weekly. Feed you new Mysid culture with newly-hatched Artemia nauplii at least twice a day. Keep an eye on the wate rquality in the Mysid tank and ensure that almost all of the Artemia nauplii are comsumed with each feeding. To maximize growth and reproduction, maintain a density of approximately 10 brine shrimp nauplii/ml of water. (You should already have your brine shrimp production up and running from our previous Breeders net columns).
One of the keys to raising Mysids is to prevent cannibalism by separating the adults from the young. For laboratory studies, mysid separation is done manually by isolating the adults, transferring ovigerous (egg-bearing) females to a culture dish, and removing the juveniles with a pipette.
A better method can be devised that will automatically separate the juveniles from the adults, if you are willing to set-up a separate tank just for the adults alongside the main culture tank. Keep the top of this isolation tank exactly even with the top of the culture tank, and position an air lift tube in a corner of the adult’s tank so that it returns water to the main culture tank, while a siphon tube in the opposite corner maintains the water level (see diagram) between the tanks. The air-lift tube should be sheathed with plankton netting or nylon screen with a mesh size (800 microns) that will restrain the adults while allowing the larvae to pass through unimpeded. Likewise, the end of the siphon tube should be covered with 500 mM plankton netting that will allow newly-hatched brine shrimp to pass through but not the juvenile mysids. Adjusting the air lift so it produces a slow, gentle, steady flow of water will automatically deposit the juvenile shrimp in the main culture tank while keeping the adults isolated in the adjacent tank, thereby eliminating cannibalism.
It is best to let the population of mysids build up for a couple of generations to increase your brood stock before you begin harvesting regularly. Meaning you should start your cultures about 6-8 weeks before you need the shrimp. As an example, when your brood stock numbers equal approximately 400-500 adults, you should be able to harvest about 200 Mysis juveniles per day to feed your fish fry without concern for depleting the mysid reserves.
Harvesting Mysids
To harvest the shrimp, sweep a net through the water column over the bare glass at the center of the tank, and select the Mysids that are the best sized for your fish fry. Using nets with progressively larger mesh will allow you to gather larger Mysis nauplii that are the perfect size for your fish fry, or conversely by using smaller net mesh sizes will allow the capture of smaller Mysids. Be sure to leave at least 20% of each generation of Mysids behind to ensure your culture is self-sustaining.
One of the biggest obstacles to raising Mysids at home is obtaining a supply of shrimp to start a culture. Aquatic Indicators (see online suppliers) is one of the few companies that sell live Mysis shrimp. One large order includes approximately hundred Mysidopsis–enough for several starter cultures.
Hints and Tricks (according to Hemdal):
- Various hydroids and other “pests” can show up in the brood tank and need to be removed by stripping down that tank. These pests compete with the Mysids for food, and may actually consume juvenile Mysids.
- When productivity is low, start up a new rearing tank after seven days. The reasoning is that if there is more than a one week age difference, the older Mysids will prey upon the newly added ones.
- Surplus adult Mysids can be frozen for later feeding, or added live to a large holding aquarium, as sort of a “rainy day fund”.
- The best way to remove larval Mysids from the brood tanks is by siphoning them out. With practice, an aquarist should be able to siphon out the babies at a rate of better than 20 per minute. The trick is to avoid wasting time trying to siphon out three or four day old babies, they are just too fast. Focus on the smaller one or two day old ones that are positioned on the glass of the aquarium. Free-floating babies are able to escape the siphon in any direction, making them harder to capture. Mysids crawling along the glass can only escape along a 180 degree plane, away from the siphon.
- Although time consuming, productivity in the brood tanks can be enhanced by selectively removing most of the male Mysids. This reduces predation of the larva as well as the amount of Artemia needed as food for the breeders. With a small net, capture the majority of the Mysids which do not show the female’s white brood pouch. You may remove some non-breeding females with this method, but the majority will be males. Even a 10:1 ratio of pouched to non-pouched mysids will produce many offspring.
- Some public aquariums have developed an easy larval separation technique: Separate mysids based on size using different mesh size net material. In most cases, this simply consists of capturing the entire contents of a brood tank in a standard fine mesh white aquarium net. This material is then rinsed through a standard green mesh aquarium net into an empty rearing tank. The adult mysids remaining in the green net are returned to the original brood tank, and this process is repeated every few days.
Bivalve larvae
The basic reason for the success of brine shrimp and rotifers as food organisms for larval marine fish is that it is possible for the fish farmer (or hobbyist) to produce these organisms rapidly in incredible numbers at relatively little expense and effort. Additionally, these food organisms can be nutritionally enriched, which makes it possible to adjust their nutritional value to suit the needs of many different species. Copepods are a better choice, however, because they are the natural food organism with the nutritional profile and digestive characteristics best suited to most marine fish species. Copepods are not well suited to contained culture because of their relatively long life cycle, several weeks, thus are difficult to culture in the required numbers for even a modest size fish culture installation. So are there any other possibilities for larval food organisms? Is there anything else that might have the essential characteristics of proper nutritional profile, abundance on demand, and acceptability to larval marine fish? And yes, there is.
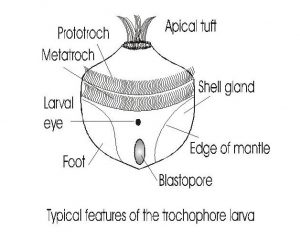
Fig 4-The trochophore larvae. The general characteristics of the trochophore larvae are illustrated in this diagram. The trochophore is the first larval stage of chitons, scaphopods, gastropods, and bivalves. The trochophore develops directly into the juvenile stage of chitons and scaphopods, but gastropods and bivalves develop through a veliger stage before metamorphosis into the juvenile.
Veliger larva
One of the strongest contenders are the first larval forms of some marine invertebrates, in particular the trochphore [Fig 4] and veliger larva [Fig 5] of bivalve mollusks: clams, oysters, scallops, and mussels. These mollusks are produced commercially as aquacultured food organisms and the techniques for spawning and rearing them are well known. In fact, there is a vast, worldwide literature on the aquaculture of bivalves, but relatively little on use of the larval forms as food organisms for larval marine fish.
The negative factors associated with use of larval bivalves in marine fish culture are that, in an inland based facility, it requires a complete system for the culture of different macro organism, including algae culture, just for production of a larval food. And the swimming behavior of the trochophore larvae is more like a rotifer than a copepod nauplius and may not stimulate some larval fish to feed.
However, there are some strong positive aspects to this source of food organisms for larval fish. The size of the larvae from various species of oysters, scallops, clams, and mollusks ranges from roughly 30 to 60 mM, a range of size that is acceptable as a first food organism for many fish [Fig 6]. Cultured oysters produce a trochophore that at 50 mM is about one fourth the size of a rotifer and one tenth the size of brine shrimp nauplii. Also the nutritional profile of the trochophore larvae can be influenced by the food fed to the adults as well as the algae fed to the early trochophore. The larvae of cultured oysters may contain 15% 20:5n3 (EPA) and 15% 22:6n3 (DHA) fatty acids. This is better than most nutritionally supplemented rotifers and brine shrimp since they have no low-n fatty acids, which are not normally found in wild fish larvae. The number of larvae that can be produced from the spawn of a single female bivalve ranges from 15 million, for an American oyster, to 55 million from a Pacific oyster, to 170 million from a scallop (Tamura, 1970). The adult bivalves are relatively easily maintained in basic closed systems, they are filter feeders so feeding is easy although particulate filtration may be a bit labor intensive. Most cultured species of bivalves can be spawned on demand. Temperature manipulation and sometimes a simple chemical shock is all that it takes to induce spawning. (I used a little vodka once to start spawning in a tank of oysters.)
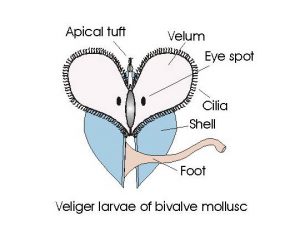
Fig 5 -The veliger larvae. The veliger stage is different in the gastropod and bivalves. The development of the vestigial shell is different, both have a velum, but the torsion that creates the single spiral shell of gastropods and development of the bivalve shell of oysters and other bivalves changes the shape and structure of the veliger larvae of these two huge Classes of mollusks.
Obtaining Bivalves
There are three ways to obtain bivalves for spawning purposes. If one lives near the sea, it is usually possible to collect oysters and mussels from the shoreline, clams from the mud flats, and scallops from the grass beds. It may be possible, depending on seasonality and latitude, to spawn the particular species at the time of collection, or to hold and feed the adults with cornstarch (or an algae feed) to condition them for spawning in a few weeks (Utting, 1993). The last possibility is to simply purchase supplies of cryopreserved embryos and early larvae of oysters and clams, Trochofeed (Cryofeeds Ltd., Canada. (Chao, et. al., 1995). This method has the advantage of providing live trochophore larva at just the right stage of development (15 hours old, ciliated and free swimming without shell formation) at any time that they are required. It takes the spawn of about 240 carefully cultured and spawned oysters to produce a billion trochophore larva. The larva are collected and processed and then frozen in liquid nitrogen at –196oC. They are kept sealed in polyethylene straws at densities of 15 and 50 million per straw and can be thawed and shipped at any time.
Within a few days of the spawn the trochophore larvae become veligers with a velum and a vestigial shell. They settle and attach to a substrate in about 10 to 15 days. The veliger stage is not as suitable as the trochophore for larval fish food because of the development of the shell, but larger larvae may well feed upon them. Under certain situations, for certain high value species, for example, the use of trochophore larvae in marine tropical fish culture might be quite useful.

Fig 6 -Brine shrimp, rotifer, and oyster trochophore larvae. Number 1 is the newly hatched nauplius of a brine shrimp. Number 2 is a rotifer and number 3 is a group of six oyster trochophore larvae. These images were all taken from the same photomicrograph of a mixed culture so the size of each organism is in exact proportion to the other two organisms.
Online suppliers of Mysid Cultures:
- Aquatic Research Organisms, Mark Rosenqvist, 1-800-927-1650
- Chesapeake Cultures, Elizabeth Wilkens, 1-804-693-4046
- C-K Aquaculture, 1-318-797-8636
- Aquatic Indicators, Ray Less, 1-904-829-2780, (Commercial accounts only), http://www.zooplankton.com/mysid/mysid.htm
References
- Bowman, T. 1964 Mysidopsis almyra, a new estuarine mysid crustacean from Louisiana and Florida. Tulane Studies in Zoology, 12, 15 18.
- Chao, N. H., Lin, T. T., Chen, Y. J. and Hsu, H. W. 1995 Cryopreservation of late embryos and early larvae of oyster and hard clam. In: Larvi’ 95 – Fish and Shellfish Larviculture Symposium. Lavens, P., E. Jaspers, and I. Roelandts (Eds.). European Aquaculture Society, Special Publication NO. 24, Gent, Belgium, p 46.
- Domingues, P., Turk, P.E., Andrade, J.P., Lee, P.G. 1998 Pilot-scale production of mysid shrimp in a static water system. Aquaculture International, 6, 387 402.
- Hanlon, R.T., Turk, P.E., Lee, P.G. 1991 Squid and cuttlefish mariculture: an updated perspective. Journal of Cephalopod Biology, 2, 31 40.
- Hemdal, Jay. Raising Mysid Shrimp as a Home Aquarium Food. Seascope 2000.
- Kuhn, A.H., Bengtson, D.A., Simpson, K.L. 1991 Increased reproduction by mysids (Mysidopsis bahia) fed with enriched Artemia spp. nauplii. American Fisheries Society Symposium, 9, 192 199.
- Lussier, S.M., Kuhn, A., Chammas, M.J., Sewall, J. 1988 Techniques for the laboratory culture of Mysidopsis species (Crustacea: Mysidacea). Environmental Toxicology and Chemistry, 7, 969 977.
- Miller, D.C., Poucher, S., Cardin, J.A., Hansen, D. 1990 The acute and chronic toxicity of ammonia to marine fish and a mysid. Archives of Environmental Contamination and Toxicology, 19, 40 48.
- McKenney, C.L. 1996 The combined effects of salinity and temperature on various aspects of the reproductive biology of the estuarine mysid, Mysidopsis bahia. Invertebrate Reproduction and Development, 29, 9 18.
- Mauchline, J. 1980 The biology of mysids and euphausids. In: Advances in Marine Biology. Part 1. The Biology of Mysids, Vol. 18 (eds J.H.S. Blaxter, F.S. Russel & C.M. Yonge), 1 369. Academic Press, London.
- Price, W.W. 1976 The Abundance and Distribution of Mysidacea in the Shallow Waters of Galveston Island, Texas. PhD Thesis, Texas A & M University, College Station, TX.
- Reitsema, L. & Neff, J.M. 1980 A recirculating artificial seawater system for the laboratory culture of Mysidopsis almyra (Crustacea; Pericaridea). Estuaries, 3, 321 323.
- Tamura, T. 1970. Marine Aquaculture. National Science Foundation. Translation from the Japanese of the revised and enlarged second edition. 1966.
- Utting, S. D. 1993. Procedures for the maintenance and hatchery-conditioning of broodstocks. World Aquaculture, 24(3): 78-82.




To maximize growth and reproduction, maintain a density of approximately 10 brine shrimp nauplii/ml of water.
That seems a lot. 10,000 brine shrimp per litre?