
The nitrogen cycle plays a highly important role in a closed environment like that of an aquarium. Due to its presence, it is possible keep the fish and invertebrates alive, in a small viable space, therefore it is fundamental to learn to know it, mainly in respect of the life forms that we nurture.
Until a few years ago, it was thought that the nitrogen cycle in its complexity, was a complete linear process. However, most recent scientific discoveries have greatly revolutionized our well-established knowledge on the nitrogen cycle and on the micro-organisms involved in such processes. As a matter of fact, the global cycle of nitrogen in the environment, particularly in that of marine, has been integrated with at least three new links which include:
- the oxidation of ammonium by a particular group of micro-organisms, the archaeabacteria (AOA);
- the anaerobic reduction of nitrates into ammonium ion (DNRA);
- the anaerobic oxidation processes of ammonium (ANAMMOX).
In the first part of this article, I will try to review the essential and more predominant aspects of the nitrogen cycle: the transformation processes of the main components (atmospheric nitrogen, ammonium ion, nitrite, nitrate) and the role played by the bacterial species involved.
In the second part, new ways will be explored with particular reference on the role of bacteria, focusing on the implications that these new discoveries have brought in the global cycle of nitrogen.
The Canonical Nitrogen Cycle
Nitrogen (N) is an essential nutrient for all organisms, and it is a critical element of protein, vitamins and DNA, and is important in biochemical structures and process that define life.
Nitrogen exists in different states of oxidation and in many chemical forms and is quickly converted by the microorganisms both on earth and the sea.
In the marine environment, nitrogen is present in 5 forms:
- Gaseous nitrogen (N2), stable molecules that require specialized enzyme systems (present in some types of bacteria) for fixation and later use;
- Ammonium ion (NH4+), the most reduced natural specie of nitrogen, and the most biologically available in an oxygen-less environment;
- Nitrate ion (NO3–), the most oxidized form of nitrogen and mostly usable in an aerobic environment;
- Particulate organic nitrogen (PON), organic form of nitrogen predominant in sediments;
- Dissolved organic nitrogen (DON), a rich mixture of molecules with a wide range of composition.
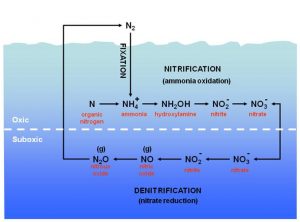
A complex network of reactions links these nitrogen forms in processes that as a whole, is called the nitrogen cycle (Figure 1). The greatest source of nitrogen comes in the form of inert gas N2 (N ≡ N), representing 78% of the atmosphere. A small part of the atmospheric N2 is fixed by particular bacteria called nitrogen-fixing (nitrogen fixation) and is reduced to ammonium ion (NH4+) which can be easily usable for other organisms. In a marine environment which inhabited by particular bacteria, ammonium is quickly oxidized to nitrate in aerobic conditions (nitrification). Nitrate is then reduced again to an N2 gas in anaerobic conditions (denitrification), thereby completing the cycle (Figure 1).
Nitrogen Fixation and Ammonification
Nitrogen fixation
The biological fixation of nitrogen can be synthetically represented by the following global formula:
N2 + 8H+ + 6e– → 2NH4+
Which means that for each molecule of atmospheric nitrogen, 2 ammonium ions are formed with the absorption of 6 electrons and 6H+, this last process tends to increase the pH.
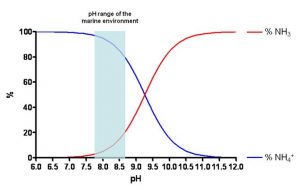
It is interesting to note that, ultimately, the ammonium ion in the water is in balance with ammonia (NH3) based on the following stoichiometry:
NH3 + H2O ↔ NH4+ + OH–
The concentration of the two chemical species relies largely on the pH, in short, the higher the alkalinity, the larger will be the quantity of ammonia; or proportion-wise, the lower the pH is (more acid), the larger will be the quantity of ammonium ion (less toxic than ammonia). As can be seen in Figure 2, in an average range of pH in the seawater, the percentage of NH4+ is higher (82-97%) compared to that of NH3 (3-18%).
As we have previously stated, the atmospheric nitrogen N2, before being incorporated into the biological molecules, has to be reduced to NH4+, through a series of reactions called biological fixation of nitrogen. Such reactions are catalyzed by a particular enzyme, nitrogenase, which is present in some nitrogen-fixing bacteria belonging mainly to Cyanobacteria phylum. One of the peculiar characteristics of this enzyme is that it comes irreversibly inhibited by the molecular oxygen (O2); and since fixation is a process that happens in an aerobic environment, it creates an apparent paradox. In reality, cyanobacteria are able to negotiate the activities of nitrogenase, an enzyme which is essentially anaerobic, with the inevitable presence of oxygen (resulting from photosynthetic processes), through not yet well-known mechanisms. In the marine environment, the nitrogen-fixing bacteria (some of which also belong to Clostridium and Azobacter genera) can be found both in free form and in symbiosis with other organisms (ex. Sponge).
But what is the source of nitrogen in an aquarium? Certainly, the biological fixation of nitrogen is an extremely important process in the ocean, but it has a limited role in the tank.
The main source of nitrogen is obtained from the nourishment both of the fish and invertebrates, particularly in the form of protein and single amino acids, assuming they are directly administered into the tank. Even minor vitamins and other molecules like the DNA, contain nitrogen but the quantity is decisively less than that of protein’s. In proteins, nitrogen forms a part of the framework and of some single amino-acids’ lateral chains, as the tryptophan, asparagin, glutamine, lysin, arginine, histidine.
The oxidative degradation of amino acids leads to the release of ammonia nitrogen into the tank. In what way? On one hand, the protein ingested by the fish or by the other organisms are broken down into single amino acids. In turn, amino acids can be used to build new proteins within the organism or be oxidized to supply energy. The degradation of amino acids by the animals leads to the elimination of varied by-products. For example, the fish release nitrogen as ammonia, while the majority of organisms may release it in the form of uric acid (fowls, reptiles), or urea (humans). On the other hand, in the presence of a strong organic charge, protein and amino acids in waste products, in sediments and in organic decay are decomposed in a process called ammonification, carried out by particular decomposer bacteria which release ammonium into the water by degrading the aminoacidic nitrogen.
Nitrification
Nitrification occurs in two distinct stages:
- oxidation of ammonium to nitrite (nitrosation) and
- oxidation of nitrite to nitrate (nitration).
1) Nitrosation: in the first stage, ammonium ion is oxidized to nitrite in two steps:
- The first step is catalyzed by the enzyme, monooxygenase which forms the hydroxylamine by using O2 as oxidant:
2NH4+ + O2 → 2NH2OH + 2H+ - In the second step, hydroxylamine is oxidized to nitrite by the enzyme hydroxylamine-dehydrogenase:
2NH2OH + 2O2 → 2H+ + 2H2O + 2NO2–
2) Nitration: the oxidation of nitrite to nitrate, which occurs through the activity of the nitrite oxidase enzyme, completes the process of nitrification:
2NO2– + O2 → 2NO3–
The conventional view of nitrification occurs in the presence of oxygen and anticipates the oxidation of ammonium to nitrate based on the following global synthetic formula (see Figure 1):
2NH4+ + 4O2 → 4H+ + 2H2O + 2NO3–
But who directs the music? The metabolic work of nitrification is entrusted to two groups of nitrifying bacteria:
- bacteria which oxidize ammonium (ammonia-oxidizing bacteria or AOB), also called nitrous bacteria. They belong chiefly to the Nitrosococcus and Nitrosomonas species;
- bacteria which oxidize nitrite (Nitrite-oxidizing bacteria or NOB) also called nitric bacteria. They form a part of the Nitrobacter, Nitrococcus and Nitrospina species.
The nitrifying bacteria are generally obliged aerobes and obliged chemoautorophs because they directly use CO2 as a source of carbon, while organic substances can be toxic.
Denitrification
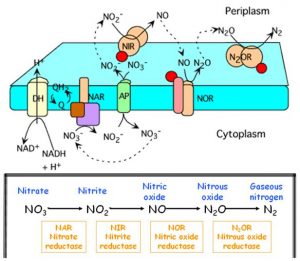
Here I describe the four stages of denitrification process in detail. The oxidation state of nitrogen is indicated by the enclosing parentheses, after the names of chemical species.
- Reduction of nitrate (+5) to nitrite (+3). This reaction is catalyzed by nitrate reductase (NAR) which exists in the (internal) cytoplasmic part of bacterial membrane. Nitrate is carried within the bacterial cell by a specialized carrier (AP in Figure 3), defined as antiport because it exchange ion nitrate upon entry with the nitrite which is produced in the reaction and must be carried to the (external) periplasmatic space for the subsequent reaction.
2NO3– + 4H+ + 4e– → 2NO2– + 2H2O - Reduction of nitrite (+3) to nitric oxide (+2). The nitrite which is now at the periplasmatic space is reduced by nitrite reductase (NIR), releasing nitric oxide (NO). NO is a remarkably important molecule, from the bacteria to humans (but this is another story).
2NO2– + 4H+ + 2e– → 2NO + 2H2O - Reduction of nitric oxide (+2) to nitrous oxide (+1). NO is reduced by nitric oxide reductase (NOR) to nitrous oxide (also called nitrogen protoxide otherwise known as the laughing gas). Both oxides represent a strong stimulus to the reductase synthesis in the presence of nitrates and under anaerobic conditions.
2NO + 2H+ + 2e– → N2O + H2O - Reduction of nitrous oxide (+1) to gaseous nitrogen (0). The last reaction in the denitrification process is the reduction of nitrous oxide to molecular nitrogen in gaseous form by the nitrous oxide reductase. This reaction should complete the denitrification process and conclude the nitrogen cycle.
N2O + 2H+ + 2e– → N2 + H2O
Denitrification is one of the key processes within the nitrogen cycle and anticipates the reduction of nitrates to gaseous nitrogen, passing through nitrite, nitric oxide (nitrogen monoxide) and nitrous oxide (nitrogen protoxide).
The global reaction of denitrification (without considering the organic molecular degradation eventually associated) can be synthesized with the following formula (for details see Figure 1):
2NO3– + 12H+ + 10e– → N2 + 6H2O
Denitrification is mainly a heterotrophic option and occurs in anaerobic conditions. A wide range of bacteria called precisely denitrifying bacteria are able to carry out the entire sequence of reactions, being equipped with a complete enzyme apparatus.
The denitrifying bacteria are able to accomplish the anaerobic respiration of nitrates by using the nitrate in place of oxygen, as acceptor of the electrons released during the respiratory process. These bacteria possess special enzymes (Figure 3), as the nitrate reductase (NAR) and nitrite reductase (NIR), which allows the electrons to flow towards nitrate or nitrite, in the absence of oxygen. They are flexible enzymes which form in the cellular membrane only under anaerobic conditions: as a matter of fact, a part of NAR, the reductase synthesis is inhibited in the presence of oxygen.
Some bacterial species of the Pseudomonas, Thiobacillus, Paracoccus and Naisseria classes, are considered denitrifying.
New Developments and New Prospects
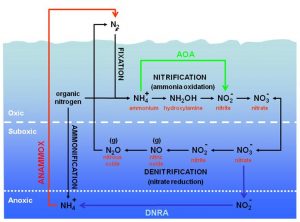
The previous description represents a well-known scenario for a long time. In the course of the recent years however, our references concerning the nitrogen cycle have drastically changed to the extent that the principle of closed linear cycle itself is being questioned. This is because new reactions have been discovered and consequently new microorganisms that make the entire nitrogen cycle even more complex and twisted (Figure 4). In the second part of this article, I will try to clarify some important ways which will be inserted within the canonical nitrogen cycle:
- Ammonium oxidation by a particular group of microorganisms, the archaeabacteria (AOA)
- The anaerobic reduction of nitrates to ammonium (DNRA):
- The anaerobic oxidation processes of ammonium (ANAMMOX)
AOA: Ammonium Oxidizing Archaeabacteria
Recently, new important components of nitrogen cycle, which form the part of the richer and diffused group of micro-organisms in the planet, the archaeabacteria, have been identified. In spite of the group’s evolutive line being unclear, the archaeabacteria (Archaea or Archeobacteria) combined with the eukaryotes and with eubacteria, are some of the fundamental domains of the living beings.

Figure 4.Nitrogen cycle integrated with recently discovered reactions. Nitrogen oxidation states are pointed out.
The archaeabacteria, like the bacteria, consist of single cells without nucleus and in the past they were classified as prokaryotes together with the bacteria. Based on the DNA analysis, the archaeabacteria were re-grouped into three phyla: Crenarchaeota, Euryarchaeota and Korarchaeota. The Euryarchaeota bacteria are the most prominent and they include methane producers and holophiles. The Crenarchaeota bacteria include thermophilic microorganisms, while the Korarchaeota bacteria are less known because only their DNA is recognized but no microorganism has so far been isolated. Originally, it was thought that the archaeabacteria were just inhabitants of a harsh and most hostile environment on the face of the earth. The thermophiles can grow at a temperature higher than 100°C, the psychrophiles are those which grow at temperatures lower than -10°C, while the acidophilus and the alkaliphiles grow in extremely acidic or alkaline environments, respectively. Finally, the halophiles prefer the highly saline environment. Today, we know that archaeabacteria are present in all habitats: for example the Crenarchaeota bacteria are considered ubiquitous components of zooplankton.
In 2004, a particular gene called the ammonium mono-oxygenase (amoA) was discovered in marine Crenarchaeota, indicating the capacity to oxidize ammonium. The definite and convincing link between this new gene and the ammonium oxidation in archaeabacteria has been recently established in Crenarchaeota, the Nitrosopumilus maritimus, which was isolated from the water of aquarium. N. maritimus is chemoautrophic: as a matter of fact it grows with bicarbonate as the only source of carbon (organic carbon inhibits its growth) and converts NH4+ in NO2– (green line in Figure 4 and Figure 7). Other archaeabacteria have been successively identified with this property and have been named as Ammonium oxidizing archaeabacteria (AOA). An accurate analysis of the AmoA gene in many archaeabacteria has revealed diverse isoforms of this gene, each one is associated to a microorganism which is present in different habitats (with little overlapping, for example, between sediment and water column). Symbiont archaeabacteria have also been identified, like for example the Cenarchaeum symbosiosum, a symbiont Crenarchaeota with a sponge. Surprisingly, it was observed that this archaeabacteria is not able to produce hydroxylamine as intermediate reaction (see the Nitrosation process in the BOX 2) indicating that ammonium oxidization occurs with a mechanism which is different from that of the classic nitrification. Finally, the most recent studies conclude that the majority of Crenarchaeota are AOA’s and AOA’s are microorganisms whose presence is numerically predominant in the ocean.
DNRA
During the recent years, the anaerobic reduction of nitrates/nitrite or, DNRA (acronym for Dissimilatory Nitrate/nitrite Reduction to Ammonium) has stirred up a certain interest as a relevant reaction both in the terrestrial and marine eco-systems. The reaction has been described in anoxic sediments and in the presence of bacteria of the Thioploca and Thiomargarita species. Both types of bacteria are able to concentrate nitrates within their own cells for the subsequent oxidation of sulphur-containing compounds in reduced form. In this way, they are able to reduce nitrate to ammonium passing through nitrite as an intermediary compound (blue line in Figure 4 and Figure 7). This reaction, although still needing clarification, would potentially supply nitrite and ammonium to the ANAMMOX reaction (see subsequent paragraph) in anoxic sediments.
ANAMMOX: anaerobic ammonium oxidation
As we have previously seen in the description of AOA and nitrification stages, ammonium oxidation is a strictly aerobic process. In reality, we have also seen that ammonium can be generated in hypoxic and anoxic environment (for example in sediments) through the re-mineralization of organic nitrogen process (ammonification), and/or the anaerobic nitrite reduction (DNRA). For many years, it has been thought that ammonium is inert in anaerobic conditions, which is to say, useless for living things. The problem, however, is that no bacteria that are able to metabolize ammonium without oxygen have been identified, especially due to the technical difficulty of cultivating in the laboratory bacterial strains with these characteristics. In 2008, many of these difficulties have been overcome and some laboratories were able to identify, cultivate and characterize some types of ANAMMOX bacteria (Acronym for ANaerobic AMMonium OXidation) which are capable of oxidizing ammonium to gaseous nitrogen (N2) (red line in Figure 4 and Figure 7) by using nitrite as electron acceptor, instead of oxygen.
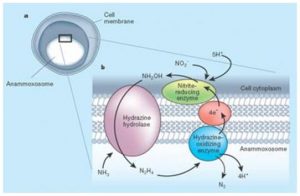
The first bacteria that are isolated in a marine environment belongs to the Scalindua species (Sc. sorokinii) although a probe regarding the presence of other species like Brocadia and Kuenenia, is being conducted. The common characteristic of these bacteria, unique in its class, is the presence of a specialized organelle called anammoxosoma which is surrounded by a particular lipid (fat) that contains hydrazine oxide reductase, an exclusive enzyme which is able to combine nitrite and ammonium is a single step (Figure 6). These bacteria use a rather complex mechanism that involves hydrazine as an intermediary. However, the following reaction, which is incomplete and stoichiometrically unprecise, can suggest the idea of an ANAMMOX process.
NH4+ + NO2– → N2 + 2H2O
This reaction has been described for the first time in sediment samples taken from particular marine ecosystems (the Black Sea, for example). It has been observed that in those experimental conditions, the ANAMMOX process was responsible for the loss of 30-50% of inorganic nitrogen from the sea (in the form of N2), significantly making it of the same level as the classic denitrification. But to analyze it closely, if we associate the above-mentioned DNRA process with ANAMMOX, we have a real and actual anaerobic denitrification, clearly not canonical. Indeed, DNRA supplies nitrite (as a reaction intermediary beginning from nitrate) and ammonium (the latter having been obtained also through organic nitrogen ammonification), and ANAMMOX transforms everything into gaseous nitrogen. A lovely and good denitrification, clearly with different mechanisms and bacterial strains, but nevertheless, a denitrification.
The question now is: under analogous conditions, can ANAMMOX occur in an aquarium? Obviously we are not able to establish that but we can make some considerations. A driven DSB can reproduce optimum conditions for this process. In fact, nitrogen bubbles are visible in the depths of the sediment. In line with this, it has been experimentally observed (but not in an aquarium) that the higher the layer of sediment, the more pushed the anoxic condition (oxygen inhibits the reaction) and the faster is the reaction. Therefore the high efficiency of a DSB in removing nitrates can be also due to a non-canonical denitrification, besides the classic anaerobic denitrification; obviously, the eventual presence of qualified bacterial strains has yet to be defined. Figure 7 illustrates the schematic diagram of integrated nitrogen cycle with the new reactions and the areas in which they occur.
In conclusion, these constant discoveries clarify many obscure points of the nitrogen cycle but at the same time they open up new horizons and paradigms that make these processes even more complex and fascinating. On the other hand, I hope not to have further complicated the well-established ideas on nitrogen cycle, with this article. I also wish that over time, these new discoveries can be applied and integrated with the aquarium, in spite of the scarce scientific researches in this area. However, even if just theoretical, this knowledge allows us to better understand that which can occur in our tanks and nurture our passion for aquarium.
References
- Arrigo K. R. (2005) Marine microorganisms and global nutrient cycles. Nature 437: 349-355.
- Berman-Frank I., Lundgren P., & Falkowski P. (2003) Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res.Microbiol. 154: 157-164.
- Brandes J. A., Devol A. H., & Deutsch C. (2007) New developments in the marine nitrogen cycle. Chem.Rev. 107: 577-589.
- Francis C. A., Beman J. M., & Kuypers M. M. (2007) New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME.J. 1: 19-27.
- Jetten M. S. (2008) The microbial nitrogen cycle. Environ.Microbiol. 10: 2903-2909.
Acknowledgements
Marco Colasanti aka marcola62 (moderator of Reefitalia forum). A special thanks is owed to the staff of ReefItalia community for their support.
About the Author
MARCO COLASANTI, born in Rome, Italy, September 15, 1962, has a degree in biology and holds a Ph.D. in Neuroscience. He is currently a Full Professor in Cell Biology at the Department of Biology, Faculty of Sciences of the University of Rome, ITALY. Entrusted with holding courses of Cellular Biology and Laboratory of Cellular Biotechnology for the University Degree of Biology. He entered the aquarium hobby with freshwater tanks (1982) and set up his first saltwater tank in 1995. He is currently a Staff Member as a Moderator of ReefItalia, an Italian reef community. Over the last fifteen years, active in the scientific research on Nitric Oxide (NO) pathway in different models and systems, including fish and invertebrates. Co-author of more than 70 publications in international peer-reviewed ISI journals or books.



Thanks for a marvelous posting! I definitely enjoyed reading it, you may be a great author. Many things need to be done to maintain water quality and keep the fish alive. But how to lower ammonia levels in fish tank naturally?