This article was republished in collaboration with Coralscience.org. Please visit Coralscience.org to read more accessible scientific articles about coral reef research.
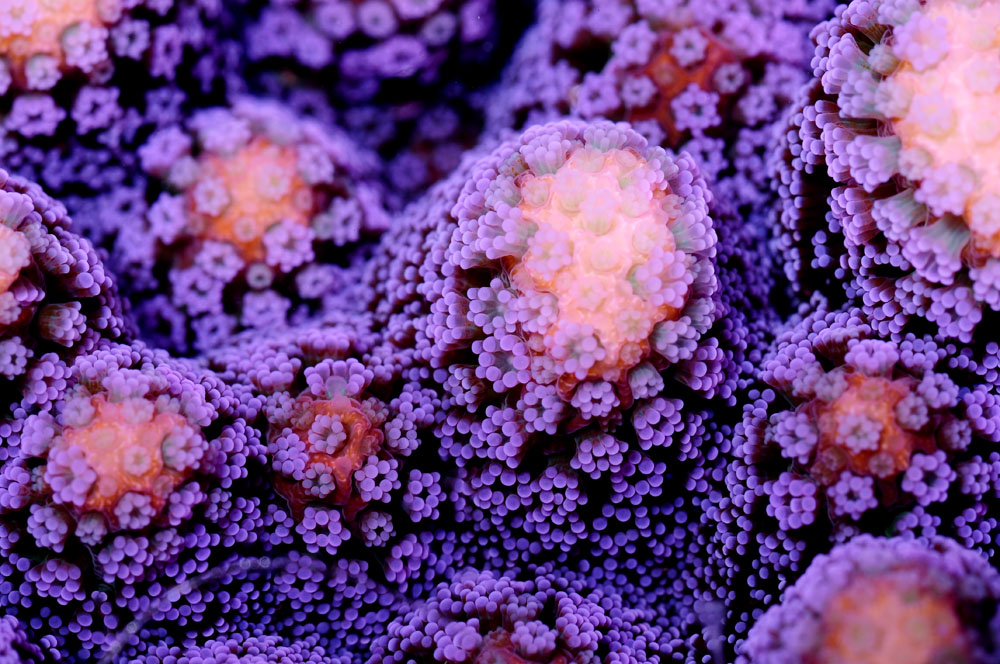
Corals, with their exquisite shapes and colours, keep mystifying us (Stylophora pistillata, photograph: Tim Wijgerde).
The marine aquarium hobby is more popular now than ever, inspired by the attractive, colourful appearance of tropical fish, corals and other invertebrates. Nowadays, the average hobbyist is able to grow countless coral species at home. To do this, a basic knowledge of coral biology is required, which can be attained by reading the available literature. This literature is the result of the efforts of several pioneers – both aquarists and scientists. In the 1960’s, our knowledge of coral biology took major leaps, due to the findings of scientists such as Leonard Muscatine, but also because of aquarists including Peter Wilkens and Jean Jaubert. Today, research on coral biology marches on, with literally thousands of scientists conducting research – both in the field and in laboratories.
People often wonder how scientists acquire certain insights. How do we know that corals live in harmony with single celled algae? How do we know that they can harness the sun’s energy? And how do we know that light alone is not sufficient for corals to grow well? To be able to answer these questions, we require a well-equipped laboratory. Only by observing, by measuring, do we slowly obtain answers to our questions.
Light is life – photosynthesis
One of the major discoveries in coral science was made in the 1920’s, by Sir Charles Maurice Yonge and his coworkers. Since the late nineteenth century, biologists knew about the existence of zooxanthellae, the single celled algae which reside in the coral’s gastroderm. After a successful expedition to the Great Barrier Reef in 1928-29, the role of these obscure algae finally became clear. Yonge found that these algae produced oxygen, a phenomenon that was also found in higher plants. By using the energy trapped in sunlight, zooxanthellae were able to convert carbon dioxide and water into organic molecules and oxygen. Later, in the 1960’s and 1970’s, biologists discovered that these organic molecules were actually translocated to the coral host tissue. By exposing corals to radioactive carbon dioxide, they observed that zooxanthellae produced radioactive glucose and glycerol, and that they leaked these into the coral’s tissue. After a lot of calculations, it became clear that these algae were able to completely meet the metabolic energy requirements of their coral host.
Even today, the photosynthetic activity of zooxanthellae is measured. This is done to find out at what intensities the algae make optimal use of the available light. If too little light is provided, the algae produce less nutrients for the coral, limiting the growth of the latter. If too much is provided, the algae may damage themselves and their host, by the production of oxygen radicals. Next to this, energy may be wasted when light becomes less effective in stimulating coral growth. It is therefore very useful to measure photosynthesis in corals, so that light energy may be used optimally for coral aquaculture.

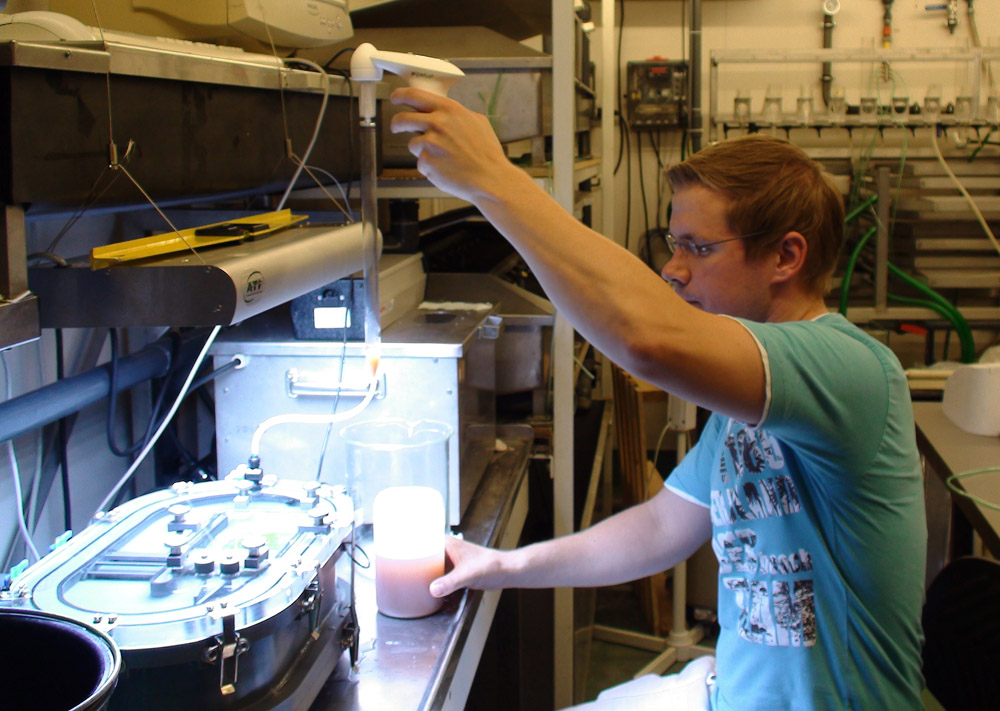
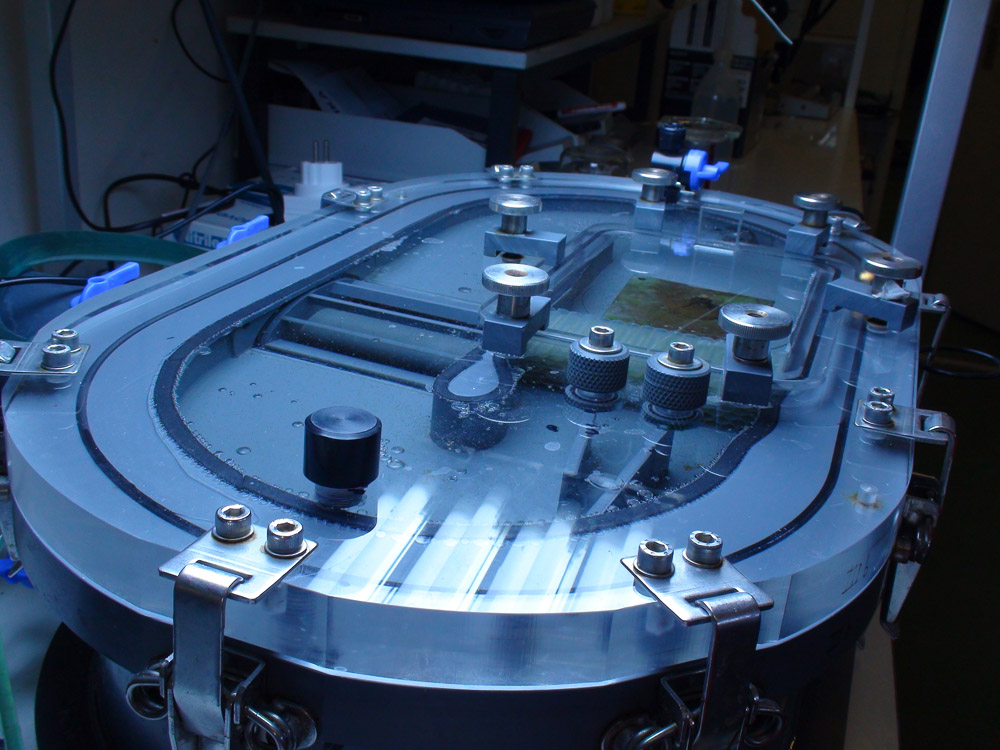
By placing corals in airtight chambers called respiration cells, photosynthesis rates can be measured accurately with optodes. These cells are equipped with water movement and temperature control (photograph: Anna Paijmans).
But how do we measure photosynthesis nowadays? This is done by placing corals inside an airtight chamber, called a respiration cell, and measuring oxygen production with optodes. These are probes which use light pulses to measure oxygen concentrations over time, allowing researchers to calculate photosynthetic rates. The higher the rate of oxygen production, the higher the photosynthetic rate, and the more nutrients zooxanthellae produce for themselves and their coral host. By gradually increasing light intensity, the relationship between irradiance and photosynthetic rates can be determined. This is visualised by a PI-curve, short for Photosynthesis-Irradiance curve.
When regarding coral lighting, people usually think: the more, the better! For several years now, it is known that this is not the case. In reality, a light source such as a T5 or metal halide fixture is sufficient for good coral growth, which can be comparable to growth rates in the wild. It is true however that corals will increase in colouration when exposed to high irradiance levels, caused by changes in pigmentation of the coral tissue and zooxanthellae. Corals are able to increase their tissue pigmentation by production of a myriad of proteins, thereby regulating the amount of light which reaches their symbiotic algae, that reside in the coral gastroderm. Zooxanthellae are able to change their pigmentation as well, allowing them to control their photosynthetic efficiency. More coral pigments cause enhanced coral colouration, and more zooxanthellae pigments lead to browner, darker corals. This adaptation of the coral host and its algal symbionts is called photoacclimation or photoadaptation. This process can be found in extreme forms in the wild. Scleractinian corals from the genera Montipora and Pachyseris, harbouring symbiotic algae, have been found at depths of up to 165 meters (550 feet)! These corals are quite pigmented as a result of photoacclimation by their zooxanthellae, resulting in a brownish appearance. Another example is the species Stylophora pistillata, which can be found in the Gulf of Aqaba at a depth of over 80 meters (267 feet), containing zooxanthellae that are so heavily pigmentated the corals appear almost black.
Breathing is essential – respiration
Not only photosynthesis, but also the respiration of the coral and its zooxanthellae can be measured. When organisms respire (breathe), organic molecules are oxidised by using oxygen, which makes it essentially the opposite of photosynthesis. Respiration is essential for plants and animals, as this yields the required chemical energy which allows their body cells to thrive. Measuring respiration is almost the same as for photosynthesis, except for the fact that the corals are incubated in total darkness. Optodes register a decrease in oxygen concentration over time, which is used to calculate respiration rates. By subtracting the total daily respiration of the coral and symbionts from the total daily photosynthesis, a certain amount of oxygen is left. This can be converted to carbon equivalents, as oxygen and carbon are consumed in a fixed ratio. The amount of daily carbon left can be used by the coral for growth, mucus synthesis and reproduction. The take home message is that corals are left with a surplus of energy for growth, defense and reproduction.
With average light intensity, good coral growth can be achieved, which can be as high as on a natural reef. Average light intensity would be an irradiance of 200 to 400 microEinsteins per meter squared per second (see text box). These irradiance levels can be achieved with either T5, metal halide, plasma or LED fixtures.
Measuring light

With a professional PAR meter, the light intensity of a light source can be determined accurately. (photograph: Tim Wijgerde)
In the marine hobby, lighting is a hotly debated subject. To quantify light, the lux unit is often used, which is the amount of lumens per square meter. Although this unit is very useful to quantify lighting equipment which is perceived by the human eye, it is of little value to the aquarium hobby or aquaculture. This is because lux and lumen are based on the green portion of the light spectrum (approximately 555 nanometers), whilst zooxanthellae, as most plants, benefit more from the red and blue portions. This is due to the fact that the photosynthetic pigments residing in zooxanthellae have the highest light absorption, and thus photosynthetic activity, when exposed to red and blue light. This principle has lead to much confusion. Light sources with little green and yellow light such as LED fixtures may seem quite dim to our eyes, while they can have a high output in the red and blue parts of the spectrum. Such a ‘weak’ light source may in fact be quite appropriate for culturing corals.
Although the human eye may not be a reliable light meter, alternatives fortunately exist. With industrial light meters, light sources can be measured accurately. A common unit of measure for light intensity is the mole or Einstein. Scientists often use the notation µmol/m2/s (or µE/m2/s), short for micromoles per meter squared per second (microEinsteins per meter squared per second). This is the amount of photons, or quanta, that hits a surface area of one square meter in a single second. This unit provides useful data for science, and can be used to perform calculations and make accurate comparisons between different conditions. The spectrum range which is used for measurement is approximately 400 to 700 nanometers, which is the portion of light that is visible to the human eye. This part of the light spectrum is able to stimulate the photosynthetic pigments residing in plants, and is therefore called Photosynthetically Active Radiation, or PAR. It is not a unit of measurement, but rather a quality that is being measured (such as distance, or time). PUR, photosynthetically Useable Radiation, is that part of the visible light which is actually absorbed by the complement of photoreactive pigments in a given plant. This will differ between plant species, and also for different clades of zooxanthellae. It is therefore not possible to assign a PUR value to a given lamp, as it depends on the organism that is receiving the light. For example, a coral species which inhabits a deep reef is exposed to dim, blue light. For such a species, a light source containing a high amount of blue in its spectrum may provide a lot of PUR. At the same time, this light source may show a low PAR value, if all other colours are emitted in low intensity.
The importance of plankton
By providing corals with zooplankton prey, in this case Artemia nauplii, their feeding capabilities can be studied (photograph: Pascal Spijkers).
Now that we know that corals can grow well when exposed to sufficient light, the question remains why corals should be fed with plankton. A review about the importance of plankton can be found under the nutrition section of this website (or download the pdf). In short, feeding corals with plankton is important as this is a source of essential nutrients. Although zooxanthellae are able to provide corals with ample energy, mainly in the form of glucose and glycerol, this is not sufficient for long-term growth. Every organism requires building blocks, for tissue growth and repair. These building blocks contain carbon, but also nitrogen and phosphorous. An athlete is very well able to exercise on a few tablets of Dextro Energy, but that person would need more to stay healthy. A daily dose of various nutrients is vital to long-term survival and health.
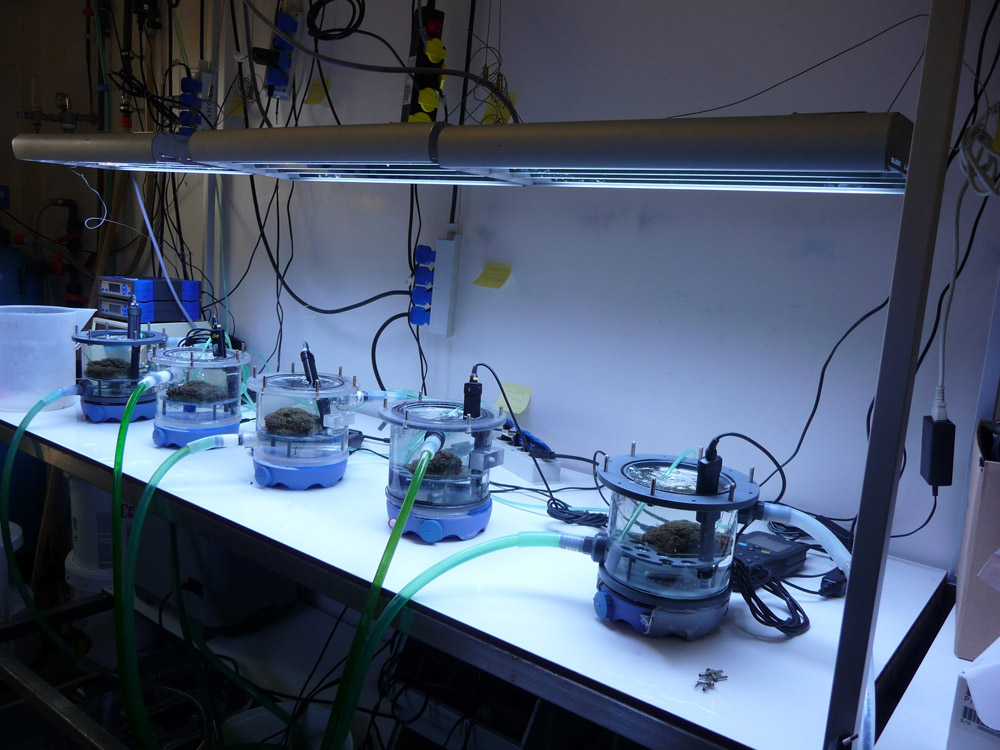
When corals are provided with ample light and zooplankton as food, they may grow optimally. But how do we know how much food is enough? Even this question can be answered by conducting experiments. At Aquaculture and Fisheries (Wageningen University, The Netherlands), a device for measuring photosynthesis, respiration and plankton capture was developed several years ago. Zooplankton (Artemia nauplii) can be dosed to this so-called flow cell with a pipette, after which the feeding behaviour of a single coral polyp or colony can be studied. With this flow cell, biologists can determine maximum prey uptake for any given species.
Go with the flow – water movement
Next to light and feeding, water movement also is of high importance to corals. Water flow is essential for food uptake, waste removal and gas exchange. For example, prey capture can be strongly influenced by the water flow rate around a coral. With the aforementioned flow cell, the water flow rate (measured in centimeters per second) can be manipulated very accurately, and the effect of flow rate on prey capture can be studied. Long-term effects of flow rate on coral growth can be determined as well, by exposing corals in experimental aquaria to various flow regimes for months at a time.
To measure is to know
It is clear that coral growth is influenced by many factors, both abiotic and biotic. Light, nutrition and water movement are examples of important (a)biotic factors. Water quality is a key fourth, but beyond the scope of this article. Not only can these factors be studied individually, interactions between these are important as well. For example, light and nutrition can act synergistically and yield very high coral growth rates. In the end, the important thing is that coral husbandry is based on scientific findings, which tell us clearly how these amazing creatures respond to different conditions. The equipment described above is only a fraction of the marine biologist’s arsenal, and is intended only to provide a look inside a coral lab.
The home aquarist may also conduct useful experiments, by manipulating light, nutrition and water movement step by step. Should you try this at home, do not change more than one factor at a time, so that some conclusions may still be drawn. When two or more factors are changed simultaneously, meaningful conclusions can only be drawn when these factors have also been studied individually. It is also important to use groups of coral fragments, called clones or ramets, to rule out chance. Meaningful experiments can lead to meaningful conclusions, which may benefit everyone. A proper understanding of coral biology is not only important to science, but also benefits coral aquaculture and the aquarium hobby.




0 Comments