
The notion that corals, like all other animals, need to feed in order to grow properly seems to have finally pervaded the aquarium hobby. Since the influential works of coral scientists such as Thomas Goreau and Leonard Muscatine, our knowledge of how corals gain nutrition has been steadily increasing. Today, we know that corals which form a mutualistic symbiosis with dinoflagellates (Symbiodinium spp.) gain most of their carbon energy from these so-called zooxanthellae (also see Wijgerde 2013a). However, carbon-rich compounds such as glucose and glycerol alone are not sufficient for corals to grow. For that, other elements such as nitrogen, phosphorus and sulfur are required. The current view is that zooxanthellae do not provide their host coral with sufficient amounts of these elements for corals to completely rely on the symbiosis. It is for this reason that corals also feed by taking up organic nutrients from the external environment. Indeed, providing corals with an external, organic nutrient source has pronounced effects on their growth. This raises important questions, such as: what do corals feed on, how is feeding affected by environmental factors, and what does this imply for the aquarium hobby and cost-efficient coral aquaculture?
The notion that corals, like all other animals, need to feed in order to grow properly seems to have finally pervaded the aquarium hobby. Since the influential works of coral scientists such as Thomas Goreau and Leonard Muscatine, our knowledge of how corals gain nutrition has been steadily increasing. Today, we know that corals which form a mutualistic symbiosis with dinoflagellates (Symbiodinium spp.) gain most of their carbon energy from these so-called zooxanthellae (also see Wijgerde 2013a). However, carbon-rich compounds such as glucose and glycerol alone are not sufficient for corals to grow. For that, other elements such as nitrogen, phosphorus and sulfur are required. The current view is that zooxanthellae do not provide their host coral with sufficient amounts of these elements for corals to completely rely on the symbiosis. It is for this reason that corals also feed by taking up organic nutrients from the external environment. Indeed, providing corals with an external, organic nutrient source has pronounced effects on their growth. This raises important questions, such as: what do corals feed on, how is feeding affected by environmental factors, and what does this imply for the aquarium hobby and cost-efficient coral aquaculture?

Corals can feed on food particles with mesenterial filaments, extensions of the stomach wall. Here, they are visible as white strands emanating from Stylophora pistillata polyps.
What do corals feed on?
Translocated nutrients from symbiotic zooxanthellae, endolithic algae and nitrogen-fixing bacteria
Under natural conditions, corals receive most of their organic carbon from the endosymbiotic zooxanthellae, and in some cases, endolithic algae which reside in the coral skeleton (Muscatine et al. 1990; Fine and Loya 2002). By using light energy, zooxanthellae (genus Symbiodinium) and endolithic algae (genus Ostreobium) convertinorganic compounds obtained from the coral and seawater (carbon dioxide, bicarbonate, ammonium, nitrate, phosphate) to organic molecules such as glucose and glycerol. This process is known as photosynthesis or photoautotrophy (from the Greek words phs, or light, autos, or self, and troph, or feeding), and it allows the zooxanthellae to feed themselves and their host coral, as the surplus of produced organic compounds is released into the coral’s cells. The inorganic waste products produced by the coral (carbon dioxide, ammonium) are then recycled by the zooxanthellae. In addition, nitrogen-fixing cyanobacteria provide zooxanthellae with ammonia, which the bacteria produce from dissolved nitrogen (N2). This nutrient exchange between corals, zooxanthellae and bacteria allows corals to grow in what is sometimes called a desert-like sea, with low nutrient availability (Muscatine 1990; Lesser et al. 2007).
A limitation of photosynthesis, however, is that it seems unable to provide corals with sufficient organic nitrogen and phosphorus to maintain tissue growth and organic matrix synthesis (see below). Therefore, corals have to feed on organic material, which is called heterotrophy or heterotrophic feeding (from the Greek words heteros, or different, and troph, or feeding). Below, I will describe the various external food sources corals can utilize. It is important to note that not every coral species may be able to use of all these sources. In addition, the degree to which corals are auto- or heterotrophic depends on environmental conditions, such as the availability of light and food particles.
Bacteria and protists
Although it is likely that corals ingest and digest viruses (femtoplankton, particle size <0.2 µm), it has been established that corals can feed on microbes such as (cyano)bacteria and flagellates. These microbes are classified as picoplankton, with a particle size of 0.2-2 µm, and nanoplankton, with a size of 2-20 µm. During a study on coral feeding, Houlbrèque et al. (2004) incubated three scleractinian corals, Stylophora pistillata, Galaxea fascicularis and the azooxanthellate Tubastraea aurea for 6 hours in flow chambers containing pico- and nanoplankton (particle size <100 m). They monitored changes in the concentrations of bacteria, cyanobacteria and flagellates during the incubation, and found that these microbes were all ingested by the three coral species. Nanoflagellates were found to be an important source of nitrogen, which is important to coral growth.
It has been known since the 1990’s that corals can feed on pelagic (free-floating) microalgae, although this has been limited to octocorals. The works of Fabricius et al. (1995a,b) have shown that the corals Dendronephthya Nannochloropsis, Isochrysis and Tetraselmis Alcyonium (Elyakova et al. 1981).
A final study that suggests corals may feed on (lower or higher) plants was carried out by Lai et al. (2013), who found that the scleractinian coral Oulastrea crispata takes up seagrass particles (Halophila ovalis). They exposed coral colonies to seagrass particles that were labeled with a stable nitrogen isotope (15N) for two hours, after which they extracted the coelenteric content of ten polyps from each colony. When a colony was found to be positive for seagrass matter, they cleaned, removed and analyzed the tissue for 15N. This revealed that O. crispata takes up Halophila ovalis particles, and possibly digests and assimilates organic nutrients obtained from this seagrass. They also found that this coral absorbed organic compounds extracted from the seagrass. This study suggests that corals living in close proximity to seagrass meadows may feed on dislodged seagrass material and seagrass exudates. Similar to the study of Leal et al. (2013), the degree to which corals can digest and assimilate seagrass material remains to be determined.
Videos of Galaxea fascicularis and Stylophora pistillata feeding on Artemia nauplii. G. fasicularis digests Artemia nauplii externally through the expulsion of mesenterial filaments, whereas S. pistillata ingests them.
Similar results have been found with octocorals such as Dendronephthya spp. and the Mediterranean gorgonians Paramuricea clavata and Corallium rubrum, which also feed on bacteria and protists (Fabricius et al. 1995a,b; Picciano and Ferrier-Pagès 2007; Ribes et al. 1999). Although bacteria comprise only a small fraction the total carbon input, they can be a major source of nitrogen.
Octocorals vary in their ability to capture and retain zooplankton, and soft corals specifically seem less adapted to this class of prey. For example, soft corals from the genera Sinularia, Sarcophyton, Cladiella, Nephthea, Dendronephthya and Paralemnalia are unable to retain larger zooplankton after capture (Fabricius et al. 1995a). For example, Dendronephthya hemprichi only captures small and weakly swimming zooplankton, such as bivalve and gastropod larvae, ostracods, amphipods, tintinnids (ciliates), polychaetes, and fish eggs. Items smaller than 300 µm are captured and taken up within 10-20 seconds, however, prey of 750 µm in size and beyond are hardly captured, and usually escape within a minute. Interestingly, when Dendronephthya spp. capture larger zooplankton, there are no signs of paralysis even after several minutes, or when these particles are captured several times over. It seems that Dendronephthya spp. and other soft corals do not possess sufficiently developed cnidocytes to effectively paralyze larger prey. Indeed, it has been found that the nematocysts-venom capsules inside the cnidocyte-of many octocorals are poorly developed (Fabricius and Alderslade 2001).
Black corals, as relatives of scleractinian corals, also have the capacity to capture and paralyze zooplankton. Laboratory experiments on Antipathes grandis show that its polyps are able to capture amphipods, copepods and chaetognaths. In the same way as for stony corals, capture occurs through the use of tentacles and mucus, after which cilia on the ectoderm transport food items to the mouth (Bo 2009). Black corals with large polyps, such as those of Antipathes andCirrhipathes spp., are able to ingest copepods of at least 1700 m in size.
Corals with larger polyps can devour small fishes whole, which is sometimes observed in the aquarium. These may be fish that are weakened due to some reason, which as a result are left defenseless to the tentacles and cnidocytes of large corals. Scolymia spp., Fungia spp. and Trachyphyllia geoffroyi are examples of corals that display this behavior.
Microalgae (phytoplankton)
Corals living in deeper waters also use detrital matter as a major source of nutrition. The deep water scleractinian coral Lophelia pertusa (but also gorgonians and black corals) captures marine snow, or detrital matter that is brought to the deep from higher oceanic layers via downwelling currents (Bo 2009; Davies et al. 2009). It must be noted, however, that too much sediment is detrimental to corals and reefs. High sedimentation literally suffocates the reef by blocking light, feeding and gas exchange (Erftemeijer et al. 2012).

Octocorals have characteristic, closely spaced pinnules which allow them to sieve phytoplankton from the water.
It has been known since the 1990’s that corals can feed on pelagic (free-floating) microalgae, although this has been limited to octocorals. The works of Fabricius et al. (1995a,b) have shown that the corals Dendronephthya hemprichi, D. sinaiensis, Scleronephthya corymbosa and Acabaria sp. feed mainly on phytoplankton, which in the laboratory includes Nannochloropsis, Isochrysis and Tetraselmis spp. The ability of octocorals to feed on phytoplankton is probably related to the narrowly spaced pinnules on their tentacles, as well as morphological and behavioral adaptations to living in strong flow (Fabricius 1995a).This finding matches well with the fact that plant-digesting carbohydrases (amylase and laminarinase) have been found in corals from the genus Alcyonium (Elyakova et al. 1981).
At present, there is also evidence that suggests scleractinian corals can feed on microalgae, contrary to previous beliefs. During a recent study, Leal et al. (2013) found that several corals were able to feed on phytoplankton, more specifically diatoms (Conticribra weissflogii, Thalassiosira pseudonana), a cryptophyte (Rhodomonas marina) and a haptophyte (Isochrysis galbana). All these algae are considered nanoplankton, with a size range of 4-12 µm. During feeding trials, six coral species were exposed to the different strains of algae, and after one hour, the corals were washed with filtered seawater and analyzed for algal DNA. The results showed that the azooxanthellate scleractinian coral Tubastraea coccinea was able to feed on C. weissflogii, T. pseudonana and I. galbana. The soft coral Heteroxenia fuscecens fed on R. marina, the scleractinian coral Pavona cactus ingested R. marina and I. galbana, and the temperate scleractinian coral Oculina arbuscula fed on C. weissflogii and I. galbana. The scleractinian coral Stylophora pistillata and the soft coral Sinularia flexibilis did not appear to feed on any of the microalgae tested.
These results indicate that each coral species has a preference for specific particle types, although it is not yet clear how this selectivity occurs. It also remains to be investigated to what extent the corals can digest these algae, since their cell walls (calcareous coccoliths or silica frustules) require the coral to produce specific acids and enzymes for digestion. Osinga et al. (2012) reported the existence of brush border enzymes in S. pistillata, which suggests that scleractinian corals can break down plant matter, although plant-digesting amylase and laminarinase were not found in stony corals in the early 1980’s (Elyakova et al. 1981). In addition, they demonstrated that the coral Pocillopora damicornis, a species related to S. pistillata (family Pocilloporidae), showed increased growth after several weeks of daily batch feeding with the microalgae Tetraselmis suecica. They did not find such a beneficial effect of Nannochloropsis sp., which seems to be in agreement with Leal et al. (2013), in the sense that each coral species may have a specific preference for and digestive capability of certain live food particles. The coral’s ability to digest certain materials may be related to the microbial consortium in the polyp’s digestive cavity (coelenteron), where different bacteria present may facilitate the breakdown of specific components using digestive enzymes.
The reason why fed corals show increased zooxanthellae densities is most likely that increased nitrogenous waste products (such as ammonium/NH4+) excreted by the coral enhance zooxanthellae growth. In turn, feeding may also coax the zooxanthellae to produce and translocate more amino acids to their coral host, which benefits soft tissue growth and organic matrix synthesis (Swanson and Hoegh-Guldberg 1998; Wang and Douglas 1999).
In addition to stimulating photosynthesis, feeding enhances calcification rates in zooxanthellate scleractinian corals. After eight weeks of zooplankton feeding (Artemia nauplii), calcification rates of Stylophora pistillata double. Various mechanisms may be responsible for this phenomenon. First of all, feeding can stimulate calcification via enhanced bicarbonate production. Feeding increases coral tissue mass, and thus the production of metabolic CO2. A part of this CO2 is enzymatically converted to bicarbonate, which can be used as a substrate for calcification. For the coral Stylophora pistillata, it has been calculated that it can acquire about 75% of its bicarbonate from its own metabolism. Second, more nutrition provides more chemical energy, directly but also indirectly by increasing the coral’s photosynthetic capacity (see above), which allows more calcium ions to be transported to the growing skeleton. Finally, feeding may stimulate calcification by enhancing organic matrix synthesis via increased supply of amino acids. The organic matrix is an extracellular protein framework which is secreted by coral cells, and is essential to skeleton formation. It provides a nucleation site for aragonite (calcium carbonate) crystals to grow, stimulating and regulating their formation (Allemand et al. 1998, 2004). As the organic matrix is rich in amino acids such as aspartic acid, feeding may enhance organic matrix synthesis and thus calcification by increasing the supply of this amino acid.
The reason why fed corals show increased zooxanthellae densities is most likely that increased nitrogenous waste products (such as ammonium/NH4+) excreted by the coral enhance zooxanthellae growth. In turn, feeding may also coax the zooxanthellae to produce and translocate more amino acids to their coral host, which benefits soft tissue growth and organic matrix synthesis (Swanson and Hoegh-Guldberg 1998; Wang and Douglas 1999).
In addition to stimulating photosynthesis, feeding enhances calcification rates in zooxanthellate scleractinian corals. After eight weeks of zooplankton feeding (Artemia nauplii), calcification rates of Stylophora pistillata double. Various mechanisms may be responsible for this phenomenon. First of all, feeding can stimulate calcification via enhanced bicarbonate production. Feeding increases coral tissue mass, and thus the production of metabolic CO2. A part of this CO2 is enzymatically converted to bicarbonate, which can be used as a substrate for calcification. For the coral Stylophora pistillata, it has been calculated that it can acquire about 75% of its bicarbonate from its own metabolism. Second, more nutrition provides more chemical energy, directly but also indirectly by increasing the coral’s photosynthetic capacity (see above), which allows more calcium ions to be transported to the growing skeleton. Finally, feeding may stimulate calcification by enhancing organic matrix synthesis via increased supply of amino acids. The organic matrix is an extracellular protein framework which is secreted by coral cells, and is essential to skeleton formation. It provides a nucleation site for aragonite (calcium carbonate) crystals to grow, stimulating and regulating their formation (Allemand et al. 1998, 2004). As the organic matrix is rich in amino acids such as aspartic acid, feeding may enhance organic matrix synthesis and thus calcification by increasing the supply of this amino acid.
Benthic (macro)algae
The reason why fed corals show increased zooxanthellae densities is most likely that increased nitrogenous waste products (such as ammonium/NH4+) excreted by the coral enhance zooxanthellae growth. In turn, feeding may also coax the zooxanthellae to produce and translocate more amino acids to their coral host, which benefits soft tissue growth and organic matrix synthesis (Swanson and Hoegh-Guldberg 1998; Wang and Douglas 1999).
In addition to stimulating photosynthesis, feeding enhances calcification rates in zooxanthellate scleractinian corals. After eight weeks of zooplankton feeding (Artemia nauplii), calcification rates of Stylophora pistillata double. Various mechanisms may be responsible for this phenomenon. First of all, feeding can stimulate calcification via enhanced bicarbonate production. Feeding increases coral tissue mass, and thus the production of metabolic CO2. A part of this CO2 is enzymatically converted to bicarbonate, which can be used as a substrate for calcification. For the coral Stylophora pistillata, it has been calculated that it can acquire about 75% of its bicarbonate from its own metabolism. Second, more nutrition provides more chemical energy, directly but also indirectly by increasing the coral’s photosynthetic capacity (see above), which allows more calcium ions to be transported to the growing skeleton. Finally, feeding may stimulate calcification by enhancing organic matrix synthesis via increased supply of amino acids. The organic matrix is an extracellular protein framework which is secreted by coral cells, and is essential to skeleton formation. It provides a nucleation site for aragonite (calcium carbonate) crystals to grow, stimulating and regulating their formation (Allemand et al. 1998, 2004). As the organic matrix is rich in amino acids such as aspartic acid, feeding may enhance organic matrix synthesis and thus calcification by increasing the supply of this amino acid.
Related to feeding on microalgae, corals may also feed on benthic algae. During a coral bleaching event in 2011, the stony corals Colpophyllia natans and Montastraea faveolata were found to feed on algal turfs and Dictyota macroalgae (Marhaver 2011). The corals extended their mesenterial filaments (protrusions from the gastroderm, or stomach lining) through the mouth or sides of the polyps, and physically contacted various types of algae. It is known that these mesenterial or gastric filaments contain cnidocytes and digestive cells, which allow corals to kill and digest neighboring corals, and their role in nutrient acquisition is becoming clearer (Wijgerde et al. 2011). By secreting enzymes, mesenterial filaments allow corals to externally digest food particles, after which specialized cells in the filaments may take up the liberated nutrients. By feeding on benthic algae, or their carbon-rich excretions, the bleached corals may have been compensating for the loss of nutrients normally gained via their zooxanthellae. Marhaver (2011), however, also states that organisms associated with the benthic algae, including bacteria, microalgae and microfauna may have been targeted by the corals.
A similar observation was made in our lab at Wageningen University, where several S. pistillata colonies showed pronounced expulsion of mesenterial filaments in areas where a biofilm accumulated due to stagnant water. As these biofilms are rich in organic compounds and bacteria, this may explain why the corals where sweeping these areas with filaments.
Seagrasses
A final study that suggests corals may feed on (lower or higher) plants was carried out by Lai et al. (2013), who found that the scleractinian coral Oulastrea crispata takes up seagrass particles (Halophila ovalis). They exposed coral colonies to seagrass particles that were labeled with a stable nitrogen isotope (15N) for two hours, after which they extracted the coelenteric content of ten polyps from each colony. When a colony was found to be positive for seagrass matter, they cleaned, removed and analyzed the tissue for 15N. This revealed that O. crispata takes up Halophila ovalis particles, and possibly digests and assimilates organic nutrients obtained from this seagrass. They also found that this coral absorbed organic compounds extracted from the seagrass. This study suggests that corals living in close proximity to seagrass meadows may feed on dislodged seagrass material and seagrass exudates. Similar to the study of Leal et al. (2013), the degree to which corals can digest and assimilate seagrass material remains to be determined.
Zooplankton
The ability of corals to feed on zooplankton has been extensively studied over the years, especially when considering scleractinian corals (Houlbrèque and Ferrier-Pagès 2009; Ferrier-Pagès et al. 2011). Generally, these corals are highly capable of capturing zooplankton owing to their powerful cnidocytes, which contain capsules loaded with neurotoxins and trapping lasso-like strings. In addition, these corals use mucus to entrap live prey. By using microscopic hairs called cilia, the coral’s polyps transport prey items (in)to the mouth.Zooplankton can also be digested externally with mesenterial filaments.
On coral reefs, the zooplankton preyed upon by stony corals include crustaceans such as copepods, amphipods, ostracods, mysids, worms such as polychaetes and chaetognaths (arrow worms), and many animal larvae. In the aquarium, many of these natural prey items are unavailable, and live or dead Artemia and Mysis are common feeds. Research has shown that Artemia nauplii, although not naturally available to corals, are a highly suitable feed, significantly enhancing coral growth (also see below).

Relationship between water flow rate and prey capture for four coral species. Left axis: Acanthogorgia vegae, Melithaea ochracea, and Subergorgia suberosa (prey concentration: 20 individuals L-1). Right axis: Galaxea fascicularis (prey concentration: 10,000 nauplii L-1). Values are means (N=2-4). For clarity, standard deviations have been omitted. After Dai and Lin (1993) and Wijgerde et al. (2012d).
Black corals, as relatives of scleractinian corals, also have the capacity to capture and paralyze zooplankton. Laboratory experiments on Antipathes grandis show that its polyps are able to capture amphipods, copepods and chaetognaths. In the same way as for stony corals, capture occurs through the use of tentacles and mucus, after which cilia on the ectoderm transport food items to the mouth (Bo 2009). Black corals with large polyps, such as those of Antipathes and Cirrhipathes spp., are able to ingest copepods of at least 1700 m in size.
Octocorals vary in their ability to capture and retain zooplankton, and soft corals specifically seem less adapted to this class of prey. For example, soft corals from the genera Sinularia, Sarcophyton, Cladiella, Nephthea, Dendronephthya and Paralemnalia are unable to retain larger zooplankton after capture (Fabricius et al. 1995a). For example, Dendronephthya hemprichi only captures small and weakly swimming zooplankton, such as bivalve and gastropod larvae, ostracods, amphipods, tintinnids (ciliates), polychaetes, and fish eggs. Items smaller than 300 µm are captured and taken up within 10-20 seconds, however, prey of 750 µm in size and beyond are hardly captured, and usually escape within a minute. Interestingly, when Dendronephthya spp. capture larger zooplankton, there are no signs of paralysis even after several minutes, or when these particles are captured several times over. It seems that Dendronephthya spp. and other soft corals do not possess sufficiently developed cnidocytes to effectively paralyze larger prey. Indeed, it has been found that the nematocysts-venom capsules inside the cnidocyte-of many octocorals are poorly developed (Fabricius and Alderslade 2001).
Gorgonians are octocorals that are generally well-adapted to capture zooplankton. It is known that several gorgonians, including the tropical species Subergorgia suberosa, Melithaea ochracea and Acanthogorgia vegae, are able to capture actively swimming Artemia nauplii in the laboratory (Dai and Lin 1993; Lin et al. 2002).
Hydrozoan corals (family Milleporidae, or fire corals, and family Stylasteridae, or lace corals), finally, carry powerful cnidocytes on their tentacles which allow them to capture zooplankton efficiently. Indeed, these corals are known to be voracious zooplankton feeders (Lewis 2006). Their nematocysts fire with such force that even human skin is sensitive to it; touching these corals causes an intense burning sensation and rashes. Unlike other corals, paralysis and ingestion of prey is taken care of by two types of polyps. Prey is stung by defensive stinging polyps called dactylozooids, whereas prey are ingested and digested by gastrozooids. Each gastrozooid is surrounded by five to fifteen dactylozooids, the latter being much longer and thinner.
Fishes
Corals with larger polyps can devour small fishes whole, which is sometimes observed in the aquarium. These may be fish that are weakened due to some reason, which as a result are left defenseless to the tentacles and cnidocytes of large corals. Scolymia spp., Fungia spp. and Trachyphyllia geoffroyi are examples of corals that display this behavior.
Other corals
An interesting coral food source are corals themselves. On the reef, corals have been found to feed on neighboring colonies, which they attack and digest externally with mesenterial filaments. This behavior may primarily be a form of effective competition between species and individuals within species (so-called interspecific and intraspecific competition, respectively), but it also provides corals with an additional food source.
Detritus
Detritus is a collective term for organic particles that arise from faeces, leftover food and decaying organisms. Detrital matter is common on coral reefs and in the aquarium, and slowly settles on the bottom as sediment. This sediment contains bacteria, protozoa, microscopic invertebrates, microalgae and organic material. These sedimentary sources can all serve as coral nutrients when suspended, especially for species growing in turbid waters. Experiments have revealed that many scleractinian corals can ingest and assimilate detritus (e.g. Anthony 1999,2000; Anthony and Fabricius 2000; Roff et al. 2009), which is trapped in coral mucus.
Although stony corals may ingest detritus when it is available, several gorgonians have been found to primarily feed on suspended detritus. For example, the Mediterranean gorgonians Corallium rubrum, Paramuricea clavata and Leptogorgia sarmentosa acquire most of their carbon as detritus (Ribes et al. 1999; Tsounis et al. 2006). This also seems true for some of their tropical counterparts, such as Menella and Swiftia spp. These gorgonians readily capture and ingest small pelleted fish feeds in the aquarium.
A gorgonian (Menella sp.) feeding on particulate organic matter of 5-800 µm in size (dry fish feed). Although technically not detritus, dry fish feed is similar as it is non-living organic material produced from animals and plants.
Corals living in deeper waters also use detrital matter as a major source of nutrition. The deep water scleractinian coral Lophelia pertusa (but also gorgonians and black corals) captures marine snow, or detrital matter that is brought to the deep from higher oceanic layers via downwelling currents (Bo 2009; Davies et al. 2009). It must be noted, however, that too much sediment is detrimental to corals and reefs. High sedimentation literally suffocates the reef by blocking light, feeding and gas exchange (Erftemeijer et al. 2012).
Dissolved organic matter
Dissolved organic matter (DOM) is an important food source for many corals. Although corals are known to excrete organic matter, mainly via mucus release, they do take up dissolved organic compounds from the water. With radioactive tracers, it was discovered that scleractinian corals take up dissolved glucose from the water. More ecologically relevant, corals can also absorb amino acids and urea from the seawater (Grover et al. 2006, 2008). Although these substances are present on coral reefs only in minute concentrations, they form a significant source of organic nitrogen. For Stylophora pistillata, amino acid uptake can account for 21% of the nitrogen budget (Grover et al. 2008), although the balance between this uptake and nutrient assimilation from other sources depends on what is available to the coral. Amino acids are important for synthesis of the organic matrix, an extracelluar proteinaceous framework which is required for skeletal growth in corals (see below). It is intriguing that corals also take up urea from the water. This indicates that corals may have adapted to the presence of higher animals on the reef, such as fish, which collectively produce large amounts of this nitrogen compound on a daily basis.
Corals do not only take up organic compounds, they also seem to detect these in the water. A common observation is the extension of coral tentacles after addition of plankton or organic substances to the aquarium water. Addition of the amino acids glycine, alanine or glutamate to the water results in tentacle extension, swelling of tissue (coenenchyme) and on occasion extrusion of mesenterial filaments (Goreau et al. 1971). Just like the human tongue has receptors to detect many substances, so too may corals have evolved receptors which recognize organic compounds such as amino acids. The ability to detect amino acids in the water may serve to perceive zooplankton, which allows corals to prepare for prey capture.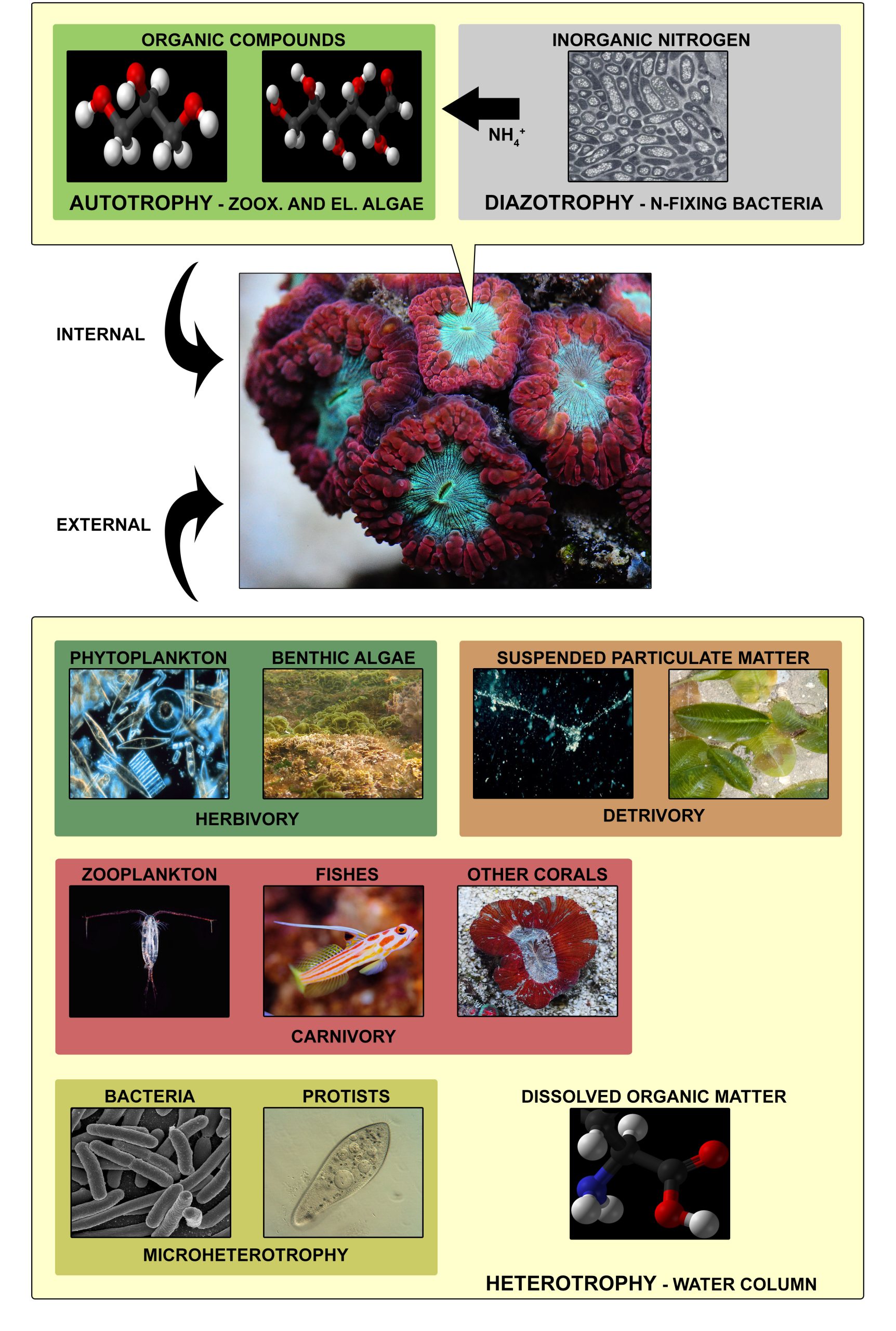
Food sources utilized by corals for energy and nutrient uptake can be divided into internal and external sources. Internal sources comprise nitrogen-fixing bacteria, which convert dissolved nitrogen (N2) into ammonia (NH3), a process called diazotrophy, and zooxanthellae, which convert the ammonia into amino acids and proteins. In addition, zooxanthellae convert carbon dioxide (CO2) to glycerol, glucose, fatty acids and amino acids via a process known as photosynthesis, a form of autotrophy. A major part of these organic compounds is translocated to the coral host cells, which use these mainly to satisfy their energy requirements. External sources comprise particulate and dissolved organic matter, which are taken up from the water column. Corals feed on phytoplankton and benthic algae (herbivory), zooplankton, small fishes and other corals (carnivory), bacteria and protists (microheterotrophy), suspended particular matter (detrivory), and finally dissolved organic matter such as urea and amino acids. This uptake of particulate and dissolved organic matter from the water column is known as heterotrophy, and the organic compounds acquired from this process are used by the coral for energy production and growth. Image credits: Ben Mills (organic compounds), Edward Palincsar (inorganic nitrogen), NOAA (phytoplankton), Toby Hudson (benthic algae), Woods Hole Oceanographic Institution and Ria Tan (suspended particulate matter), Uwe Kils (zooplankton), Tim Wijgerde (fishes, other corals), Incnis Mrsi (dissolved organic matter), NIAID/NIH (bacteria) and D. Munaretto (Protists).
To conclude thus far, it is clear that corals are able to take up organic compounds from a wide range of sources, which underscores the diverse and efficient nature of corals as omnivores.
Dissolved inorganic matter
Although this article focuses on feeding on organic compounds, corals also take up inorganic matter from the water column. I will briefly mention the most important elements taken up in inorganic form. These include, but are not limited to, inorganic nitrogen (dissolved nitrogen/N2, ammonium/NH4+ and nitrate/NO3–) and phosphorus (phosphate, HPO42-), inorganic carbon (carbon dioxide/CO2, bicarbonate/HCO3–), alkali metals (sodium/Na+, potassium/K+), alkaline earth metals (calcium/Ca2+, magnesium/Mg2+, strontium/Sr2+), transition metals (e.g. zinc/Zn2+, iron/Fe2/3+, copper/Cu2+, manganese/Mn2+), metalloids (Boron/B), and nonmetals (iodine as iodide/I– and iodate/IO3–, oxygen/O2). The uptake of inorganic nitrogen and phosphorus is due to the presence of symbiotic zooxanthellae and bacteria, which convert these to organic compounds for their growth. The uptake of (non-)metallic compounds is due to both the coral host and its symbionts. Calcium and magnesium are important for coral calcification, carbon dioxide and bicarbonate are essential to photosynthesis and coral calcification, trace elements such as zinc and iodine are used by corals and symbionts for enzyme function and possibly hormone production, and oxygen is important for respiration.
Effects of feeding on coral growth and physiology
The effects of feeding on coral growth and physiology have been well studied, and are discussed at length in recent reviews by Houlbrèque and Ferrier-Pagès (2009) and Ferrier-Pagès et al. (2011). So far, most studied have focused on the effects of zooplankton feeding, mainly Artemia nauplii, on coral growth and physiology.
Photosynthesis and zooxanthellae density
Research has shown that feeding enhances photosynthesis rates of zooxanthellate corals, by increasing zooxanthellae density and chlorophyll a content. For S. pistillata, zooxanthellae densities double within several weeks of zooplankton feeding, both at low and high light levels. The number of dinoflagellates residing in a single coral host cell also increases, with up to four zooxanthellae per coral cell. A higher photosynthetic capacity allows the coral to convert more light energy into chemical energy, which can be used for growth.
The reason why fed corals show increased zooxanthellae densities is most likely that increased nitrogenous waste products (such as ammonium/NH4+) excreted by the coral enhance zooxanthellae growth. In turn, feeding may also coax the zooxanthellae to produce and translocate more amino acids to their coral host, which benefits soft tissue growth and organic matrix synthesis (Swanson and Hoegh-Guldberg 1998; Wang and Douglas 1999).

Some examples of gluttonous feeding corals with their tentacles extended. Top left: Acanthastrea lordhowensis. Top right: Caulastraea sp. Lower left: Tubastraea sp. Lower right: Trachyphyllia geoffroyi.
Calcification and the organic matrix
In addition to stimulating photosynthesis, feeding enhances calcification rates in zooxanthellate scleractinian corals. After eight weeks of zooplankton feeding (Artemia nauplii), calcification rates of Stylophora pistillata double. Various mechanisms may be responsible for this phenomenon. First of all, feeding can stimulate calcification via enhanced bicarbonate production. Feeding increases coral tissue mass, and thus the production of metabolic CO2. A part of this CO2 is enzymatically converted to bicarbonate, which can be used as a substrate for calcification. For the coral Stylophora pistillata, it has been calculated that it can acquire about 75% of its bicarbonate from its own metabolism. Second, more nutrition provides more chemical energy, directly but also indirectly by increasing the coral’s photosynthetic capacity (see above), which allows more calcium ions to be transported to the growing skeleton. Finally, feeding may stimulate calcification by enhancing organic matrix synthesis via increased supply of amino acids. The organic matrix is an extracellular protein framework which is secreted by coral cells, and is essential to skeleton formation. It provides a nucleation site for aragonite (calcium carbonate) crystals to grow, stimulating and regulating their formation (Allemand et al. 1998, 2004). As the organic matrix is rich in amino acids such as aspartic acid, feeding may enhance organic matrix synthesis and thus calcification by increasing the supply of this amino acid.
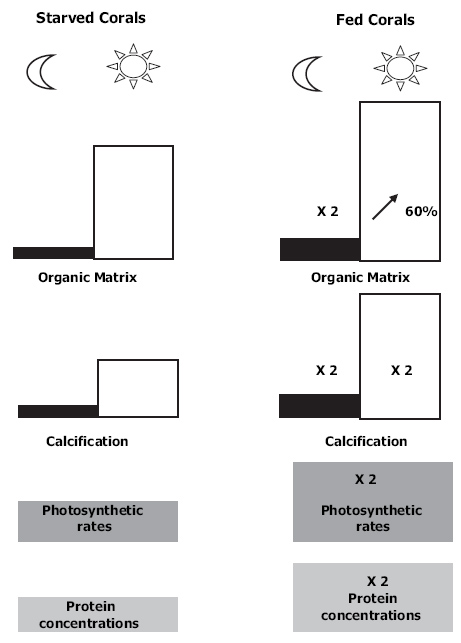
Overview which shows the profound effects of feeding on corals. Fed corals display (1) twofold greater protein concentrations and photosynthetic rates per unit skeletal surface area; (2) twofold higher dark and light calcification rates; (3) twofold higher organic matrix synthesis in the dark and a 60% increase during daytime (Houlbrèque and Ferrier-Pagès 2009).
It must be noted that heavy feeding of corals can have its drawbacks. In our lab at Wageningen UR, we studied the short-term effects of zooplankton feeding on light and dark calcification rates of the coral Galaxea fascicularis. Although feeding had little effect on growth under light conditions, in total darkness, calcification rates of fed corals were close to zero (Wijgerde et al. 2012b). Our current hypothesis is that dark calcification is inhibited by heavy feeding due to a temporary acidosis of coral tissue, caused by increased metabolic activity. During night time feeding, corals may invest energy into soft tissue growth and organic matrix synthesis, rather than calcification.
Coral tissue
Next to enhancing photosynthesis, zooxanthellae density, calcification rates and organic matrix synthesis, zooplankton feeding also increases the protein and fat content of soft tissue. Saturated and unsaturated fatty acids, as well as alcohols and sterols increase in concentration after prolonged feeding with Artemia nauplii. The increase in lipid stores allows corals to better cope with stress, most importantly bleaching. When high water temperatures induce a loss of zooxanthellae, corals can no longer perform photosynthesis and must rely on prey capture and energy reserves to survive (Grottoli et al. 2006).
What determines coral feeding rates?
Water flow
Water flow is essential to corals, for many different reasons. Next to enhancing gas exchange and promoting the removal of sediment, water flow allows corals to feed on (live) particles (Wijgerde 2013 and references therein). As the important role of water movement is quite obvious, water flow is one of the best studied factors that influence prey capture by corals.

Relationship between water flow rate and prey capture for four coral species. Left axis: Acanthogorgia vegae, Melithaea ochracea, and Subergorgia suberosa (prey concentration: 20 individuals L-1). Right axis: Galaxea fascicularis (prey concentration: 10,000 nauplii L-1). Values are means (N=2-4). For clarity, standard deviations have been omitted. After Dai and Lin (1993) and Wijgerde et al. (2012d).
Water flow has both beneficial and detrimental effects on coral feeding, depending on the rate of flow. Higher flow rates will increase the influx of food particles, and will therefore benefit feeding. However, higher flow rates will also increase the kinetic energy of food particles, which will limit the capture abilities of coral polyps. In addition, strong water flow creates drag forces, resulting in deformed polyps and decreased capture area and efficiency. These mechanisms explain why bell-shaped relationships between water flow rate and prey capture have been found for several coral species, with optima often lying between a flow range of 5 to 10 cm s-1. The graph below illustrates how flow affects the feeding rates of four different corals; the octocorals Acanthogorgia vegae, Melithaea ochracea and Subergorgia suberosa, and the scleractinian coral Galaxea fascicularis. The graph immediately shows the species-specific response to flow. The different ways in which these species respond to water flow in terms of prey capture can be explained by differences in polyp morphology (see below).
Coral size
Next to water flow rate, colony size affects heterotrophic feeding. Colony size can affect the feeding rates of individual polyps, both in negative and positive ways, due to polyp interactions within colonies. Negative effects include polyp shading (i.e. polyps covering and obstructing one another) and local particle depletion, resulting in decreased prey capture by downstream polyps (Hunter 1989). Positive effects include the generation of intracolonial turbulence and mucus secretion by upstream polyps, enhancing prey capture by downstream polyps (Wijgerde 2013 and references therein).
Although polyps in colonies can exhibit higher feeding rates compared to solitary polyps, the colony as a whole seems to become less efficient. In our lab, we found that only 7.7% of the polyps in a small Galaxea fascicularis capture zooplankton. This means that per polyp, these corals capture less food than individual polyps. This observation fits well with the decrease in relative growth of Galaxea with increasing size (Schutter et al. 2010; Wijgerde et al. 2012a). After 245 days of incubation, small Galaxea colonies show a 76% reduction (from 2.5 to 0.6 % day-1) in relative growth as compared to single polyps.
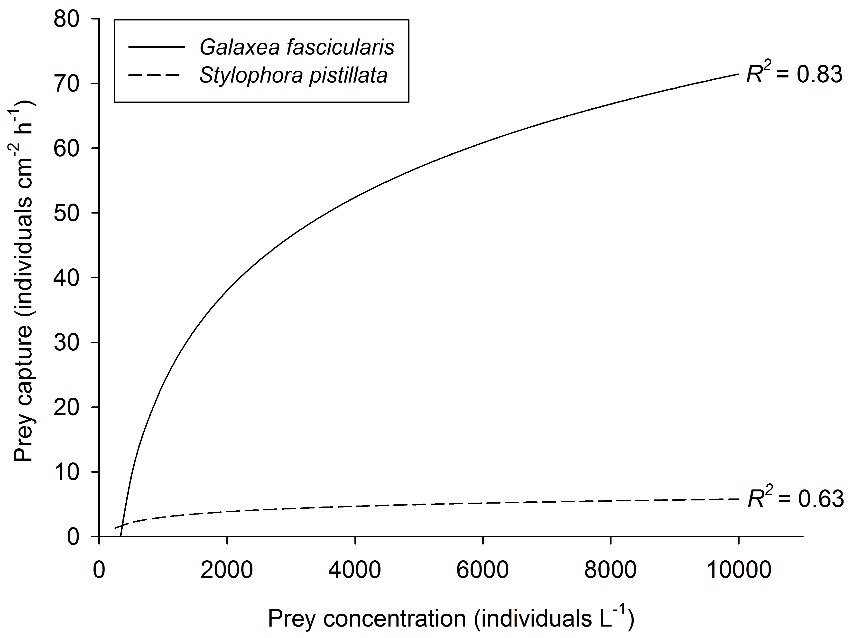
Relationship between prey concentration and prey capture for two coral species, Galaxea fascicularis (Artemia nauplii) and Stylophora pistillata (Mediterranean zooplankton). Best fit curves, N=30 and N=25, respectively. After Ferrier-Pagès et al. (2003) and Wijgerde et al. (2011a, 2012c).
Prey concentration
Prey concentration is a well-studied factor that impacts coral feeding rates. Higher prey concentrations will increase the prey encounter rate of coral polyps, which has a positive effect on feeding rates. Initially, a linear relationship between prey density and coral feeding rate is found. However, when the prey concentration gets high enough, a saturating effect is observed. This is because coral polyps are bound by a maximum number of prey they can capture, ingest and digest at a given time. This saturating effect of prey concentration is illustrated by the graph below, which shows stabilizing feeding rates of the corals Galaxea fascicularis and Stylophora pistillata with increasing prey availability.
Polyp morphology
The morphology of coral polyps is yet another factor which can influence coral feeding. For example, Subergorgia suberosa has large polyps and encounters high drag forces, causing strong currents to easily deform its polyps. This helps to explain why this species feeds in a narrow range of flow velocities, as can be seen in the flow graph above. Melithaea ochracea, in contrast, has smaller polyps and therefore encounters lower drag, resulting in less polyp deformation in stronger currents. This is probably the reason why it feeds in a wider range of flow velocities.
Although being deformed more easily, larger polyps may have a higher capacity for feeding. This is obvious in the graph above, where the larger Galaxea fascicularis polyps (~5 mm corallite diameter) capture significantly more prey compared to the much smaller Stylophora pistillata polyps (~1 mm corallite diameter). This is probably due to the fact that the polyps of G. fascicularis are able to externally digest large prey quantities.
Another result of polyp dimensions is the maximum size of prey corals are able to ingest. Small-polyped species may capture copepods and various animal larvae as maximum prey size, whereas species with large polyps (e.g. Fungiidae, Mussidae and Flabellidae) are able to consume large prey items such as fishes and shrimp.
Epizoic flatworms
The last factor which I would like to address here is the presence of epizoic acoelomorph flatworms. These flatworms are commonly referred to in the aquarium hobby as planaria, although these are actually worms from the genera Waminoa and Convolutriloba. It has been suggested that these worms can negatively affect corals by reducing the amount of available light available to the coral, and by removing the coral mucus layer (Barneah et al. 2007; Naumann et al. 2010). In addition, Waminoa compete with their host coral Galaxea fascicularis for plankton, significantly limit this coral’s feeding capacity, and steal prey from their host coral (Wijgerde et al. 2011b, 2012c).
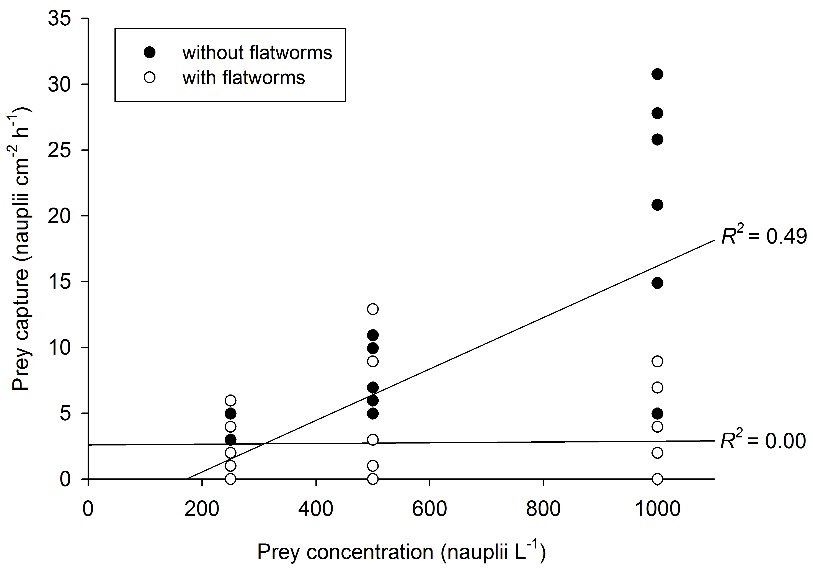
Relationship between prey concentration and prey capture for Galaxea fascicularis polyps, with (N=27) and without (N=27) epizoic flatworms. A significant, positive correlation between prey concentration and prey capture is found only for worm-free polyps. After Wijgerde et al. (2012c).
When providing G. fascicularis with prey, a positive effect of prey concentration on feeding rate is found only for worm-free polyps. When flatworms are present in high densities (~3-4 flatworms per polyp), feeding rates of G. fascicularis are limited to about 2.5 prey cm-2 h-1. Based on these findings, epizoic flatworms may be better classified as parasites rather than commensals, as their presence could negatively affect the growth and health of corals. Indeed, field evidence suggests that flatworms cause severe tissue necrosis in corals (Hoeksema and Farenzena 2012).
Obviously, the effects of water flow, coral size, prey concentration, polyp morphology and flatworms have important implications for coral aquaculture and the aquarium hobby. Below, I will discuss how this knowledge can be used to maximize coral feeding rates, and thus growth.
Feeding corals in captivity: maximizing feeding rates and growth
Armed with the knowledge described above, we can attempt to maximize coral feeding rates and growth by manipulating the aquarium environment. Below, several important strategies are described which may help the aquarist to accomplish this.
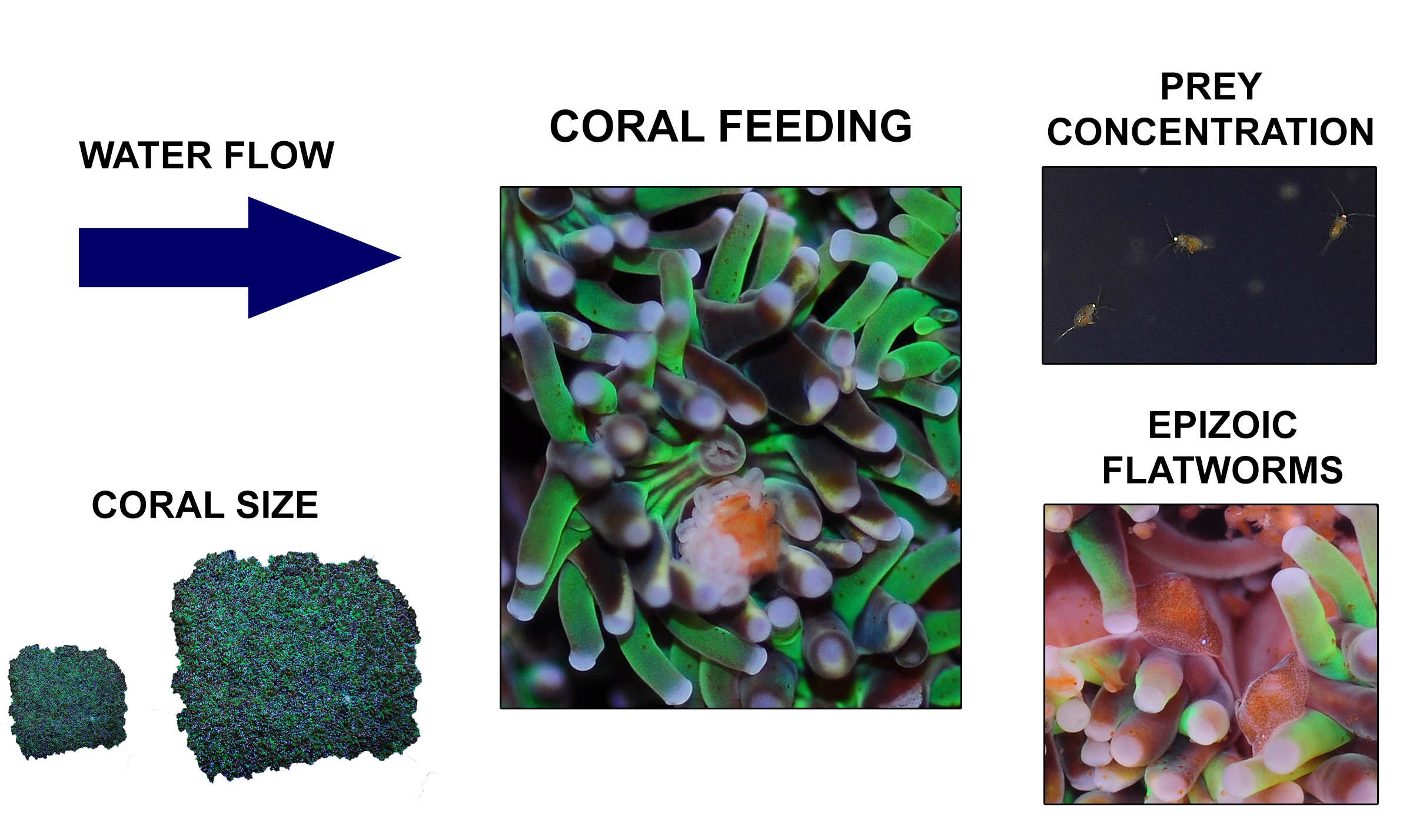
Overview of the different factors known to determine feeding rates of Galaxea fascicularis, which include water flow, coral size, prey concentration and epizoic flatworms.
Optimal water flow rate
As stated above, water flow is essential to corals, promoting growth, photosynthesis, gas and heat exchange, and sediment removal (Mass et al. 2010; Schutter et al. 2010, 2011; Jimenez et al. 2011; Erftemeijer et al. 2012). Although many corals will grow at variable flow rates, each species (and genotype within species) may grow optimally with a specific flow range. This is, at least in part, due to optimal feeding rates within a certain flow range. For example, the corals Acanthogorgia vegae, Melithaea ochracea and Subergorgia suberosa all capture zooplankton optimally at a flow rate of 8 cm s-1, possibly a reflection of their shared habitat. In addition, Subergorgia suberosa only captures food at a very narrow flow range, making the maintenance of this species highly difficult. Herbivorous octocorals may require more flow for optimal particle capture. The octocoral Dendronephthya hemprichi, which mainly feeds on phytoplankton, feeds and grows optimally within a flow range of 10 to 25 cm s-1 (Fabricius et al. 2005a).
Dendronephthya and Scleronephthya spp. produce densely packed sclerites that provide colony stability under strong water flow.
Table 1 summarizes the optimal flow rates in terms of particle capture for various coral species, which can be used for practical purposes. With a water flow meter (e.g. Swoffer flow meters), flow rates can be optimized in any aquarium, for any species for which data are available. It must be noted, however, that the data listed below may not apply to each individual within a given species, as it is known that different genotypes can behave differently (Osinga et al. 2011).
| Species | Optimal water flow rate (cm s-1) | Particle type | Comments | Reference |
|---|---|---|---|---|
| low flow | ||||
| Lophelia pertusa | 2.5 | Artemia nauplii | Purser et al. (2010) | |
| moderate flow | ||||
| Acanthogorgia vegae | 8 | Artemia nauplii | Dai and Lin (1993) | |
| Briareum asbestinum | 6-12 | not mentioned | Fabricius et al. (2005a) | |
| Eunicea tournefortis | 6-12 | not mentioned | Fabricius et al. (2005a) | |
| Galaxea fascicularis | 10 | Artemia nauplii | Wijgerde et al. (2012d) | |
| Madracis mirabilis | 10 > 5 | Artemia cysts | optimum unclear | Sebens et al. (1998) |
| Melithaea ochracea | 8 | Artemia nauplii | Dai and Lin (1993) | |
| Montastraea cavernosa | 10 > 5 | Artemia cysts | optimum unclear | Sebens et al. (1998) |
| Plexaurella dichotoma | 6-12 | not mentioned | Fabricius et al. (2005a) | |
| Porites porites | 9-11 | Artemia cysts | specific coral branches only | Sebens et al. (1998) |
| Pseudopterogorgia americana | 6-12 | not mentioned | Fabricius et al. (2005a) | |
| Subergorgia suberosa | 8 | Artemia nauplii | Dai and Lin (1993) | |
| high flow | ||||
| Agaricia agaricites | 18 | Artemia cysts | bifacial colonies | Helmuth and Sebens (1993) |
| Agaricia agaricites | 30 | Artemia cysts | vertical colonies | Helmuth and Sebens (1993) |
| Dendronephthya hemprichi | 10-25 | phytoplankton | Fabricius et al. (2005a) | |
| very high flow | ||||
| Agaricia agaricites | 18-50 | Artemia cysts | horizontal colonies | Helmuth and Sebens (1993) |
To stress the importance of flow rate, I would like to use our case study of the coral Galaxea fascicularis (Wijgerde et al. 2013). For polyps in colonies, a water flow rate of 10 cm s-1 resulted in highest feeding rates, and thus organic carbon acquisition. Using previously acquired data from our lab, a nutrient budget for this species was calculated under various flow regimes. To this end, the input versus output of organic carbon was compared. Input consisted of carbon produced by photosynthesis, and carbon gained through feeding. Output was based on respiration (i.e. the energy consumed by the non-feeding, resting animal) and excretion of organic waste. By subtracting output from input, a value known as scope for growth was obtained. Here, scope for growth was defined as the carbon available for growth, after respiration and excretion have been satisfied. Table 2 shows the scope for growth for G. fascicularis at various flow conditions, and reveals that very low and high flow rates result in negative values. This suggests that under these conditions, this coral is unable to capture sufficient prey to maintain tissue growth. Although this analysis is based on several assumptions, it allows the aquarist to select appropriate culture conditions.
| Input (g C cm-2 day-1) | Output (g C cm-2 day-1) | |||||
|---|---|---|---|---|---|---|
| Water flow rate (cm s-1) | :Photosynthesis | Feeding | Respiration | Excretion | Scope for growth | |
| 1.25 | 101.09 | 0.90 | 77.76 | 25.27 | -1.04 | |
| 5 | 101.09 | 89.00 | 77.76 | 25.27 | 87.06 | |
| 10 | 101.09 | 123.50 | 77.76 | 25.27 | 121.56 | |
| 20 | 89.86 | 30.60 | 86.40 | 22.46 | 11.59 | |
| 30 | 70.85 | 44.30 | 89.86 | 17.71 | 7.58 | |
| 40 | 70.85 | 17.70 | 89.86 | 17.71 | -19.02 | |
More flow for larger corals
Next to water flow, coral size matters. The branched structure of many corals reduces the amount of water flow and light which reaches the inner and undersides of colonies, a phenomenon known as self-shading. Therefore, larger corals require more light and water flow to maintain photosynthesis rates and gas exchange.
Feeding rates on a per polyp basis (i.e. mass-specific) can also decrease when corals grow larger, as found for G. fascicularis. All these phenomena in part explain why larger corals generally shown decreased relative growth rates (although absolute growth rates increase since there is more surface area to grow). This means that the aquarist can optimize the growth of larger corals by compensating for their size, ensuring that densely branched corals receive sufficient water flow for feeding and gas exchange. Maintaining small colonies can also be a strategy to maintain high growth in aquaculture.

By dosing cultures into a system with peristaltic pumps, natural plankton concentrations can be maintained.
High density batch feeding or continuous dosage
As feeding can have positive long-term effects on corals, providing them with a daily batch of feed is useful. An advantage of batch feeding is that it allows corals to feed quickly, without all feed being lost to the filtration systems (also see below). A difficulty with feeding, however, is determining the appropriate dosage. This depends on the biomass present in the aquarium. More corals will require more feeding, independent of aquarium volume. The species of coral and other invertebrates present will also influence the amount of feed required, as some species may require more plankton than others. A good strategy is to start with a baseline dosage of 100-1,000 prey per liter of water and inspecting the corals regularly. Loss of polyps, a lack of colony or tentacle expansion, decreases in colony size and necrosis can all be signs of starvation. Dosing amino acids before a batch feeding may also stimulate polyp expansion and predation, possibly due to molecular receptors present at the surface of the polyp ectoderm. When using batch feeding, temporary inhibition of coral growth can be prevented by feeding during daytime (see above).
Another strategy is to maintain natural plankton concentrations (in the range of 108-109 algae/microbe cells and 1-10 zooplankters per liter), by slowly pumping refrigerated cultures into the system using peristaltic pumps. Feeding timers can also regularly provide dry feeds. This strategy seems ideal for maintaining azooxanthellate corals such as Tubastraea and Dendronephthya spp.

Flatworms, here hosted by Goniopora spp. (left and right) and Euphyllia paraancora (middle), can be kept under control using natural predators.
Control over flatworm populations
As acoelomorph flatworms may have a detrimental impact on corals, it is sensible to keep flatworm populations under control by introducing natural predators to the aquarium. There is evidence that certain wrasses (e.g. Halichoerus spp.), dragonets (e.g. Synchiropus splendidus) and nudibranchs (Chelidonura varians) actively prey on flatworms (Carl 2008; Nosratpour 2008). Chemical treatment of corals with anthelmintics such as levamisole works well (for acoelomorphs), but this is laborious and could negatively affect long-term coral health.
Use of plankton-saving filtration systems
Until now, I have not addressed the impact of aquarium filtration systems on coral feeding. As most aquaria are equipped with foam fractionators (protein skimmers), with the ability to remove small particles, it logically follows that these filters will have some impact on the availability of food to corals. Indeed, when dry fish feeds or phytoplankton cultures are added to an aquarium, a part of this quickly ends up in the collection cup of the skimmer. This can be easily seen when the feed or culture has an apparent color, such as orange or green.
The obvious questions that arise from this are; how much food particles actually end up in the filtration system, and how much food will be eaten by the corals and other filter feeding organisms? To this end, a food simulator was developed within the framework of the CORALZOO research project. Using estimates of coral food capture rates, based on laboratory experiments (Wijgerde and Osinga 2007, unpublished data), and information such as coral volume (a measure of coral biomass), prey concentration, system water volume, and aquarium filtration rates, the fate of food particles added to the system can be calculated.
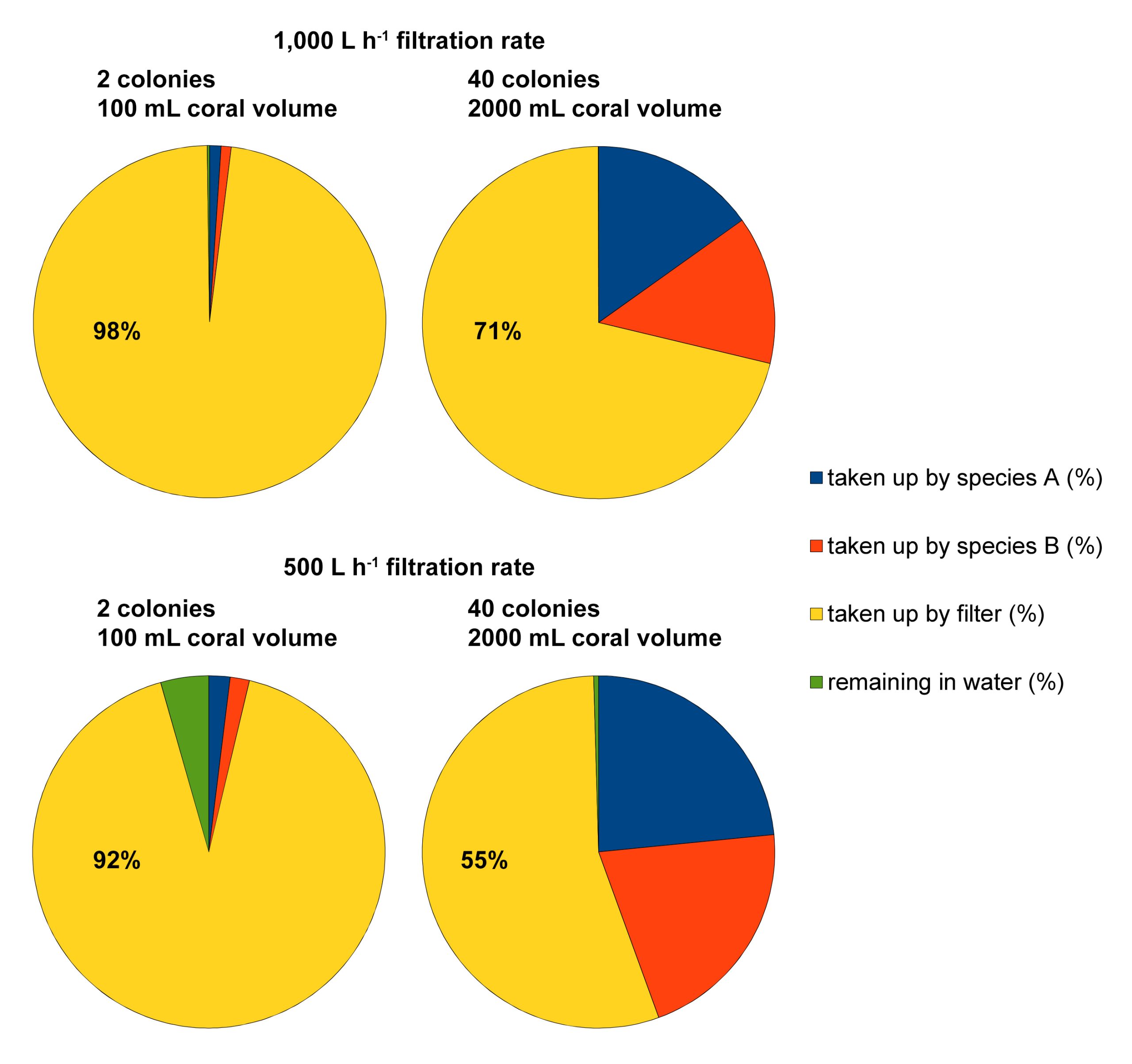
Example of four feeding scenarios calculated with the food simulator (developed by Dr Michael Kuecken, Technical University of Dresden, and Dr Ronald Osinga, Wageningen University), based on an aquarium size of 1,000L, 100/2,000 mL of coral volume with a filtration rate of 0.213 and 0.191 L h-1 mL coral-1 for Seriatopora hystrix (species A) and S. caliendrum (species B), respectively, an aquarium filtration rate of 500/1,000 L h-1, and a feeding time of 6 hours. These scenarios reveal how much particulate food may end up in the filtration system, especially when coral volume/biomass in the aquarium is low.
To illustrate how useful this program is, I have calculated two scenarios. In the first two scenarios, a 1,000L aquarium is stocked with either two (one Seriatopora hystrix and one S. caliendrum colony) or forty (twenty S. hystrix and twenty S. caliendrum colonies) corals of intermediate size (50 mL volume per colony). The aquarium filtration rate is set at 1,000 L h-1, based on a net water turnover between the aquarium and sump of 1,000 L h-1, and a protein skimmer equipped with a 1,000 L h-1 pump. Thus, the residence time of the food particles (Artemia nauplii) in the aquarium is exactly one hour. In the second two scenarios, the stocking densities are the same, but the filtration rate is halved to 500 L h-1. Thus, the residence time of the food particles (Artemia nauplii) has increased to two hours. After six hours, all scenarios are near equilibrium and the following datasets are acquired; in the first two scenarios, 98% and 71% of the food has been consumed by the filter. In the second two, these values are 92% and 55%. Although this theoretical example has several limitations, such as the assumption that all particles are removed by the protein skimmer, it does clearly show that mechanical filters (which can include biofilters and sand filters) result in a significant waste of food. This waste can be reduced by increasing the stocking density of corals, and/or by reducing the aquarium’s filtration capacity (either continuously or temporarily) to allow the corals to feed more. As filtration is required to maintain high water quality, clearly, a trade-off between food availability and water quality exists.
This trade-off between food availability and water quality can be circumvented by using plankton-saving filtration systems, which include denitrifying sand beds such as Dynamic Mineral Control (DyMiCo), and algal turf scrubbers (Wijgerde 2012a,b). As the side advantages of the protein skimmer are lost (i.e. maintenance of a high oxygen saturation and pH via aeration of the water), it is important to compensate for this, especially during night time. This can be done by aerating the water with air pumps, or by limiting the stocking density of corals and fish.
Concluding remarks
Taken together, it is clear that corals feed in many ways, making them true omnivores. This knowledge can be used effectively by aquarists, and thereby contribute to coral aquaculture and a successful aquarium hobby. By providing corals with a variety of feeds, next to sufficient light, appropriate water motion and clean water, they will grow and thrive in the aquarium. Future research will undoubtedly reveal new pathways through which these fascinating animals feed, which will allow aquarists to further refine their available culture methods.
References
- Allemand D, Ferrier-Pagès C, Furla P, Houlbrèque F, Puverel S, Reynaud S, Tambutté É, Tambutté S, Zoccola D (2004) Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C R Palevol 3:453-467
- Allemand D, Tambutté E, Girard JP, Jaubert J (1998) Organic matrix synthesis in the scleractinian coral Stylophora pistillata: role in biomineralization and potential target of the organotin tribulyltin. J Exp Biol 201:2001-2009
- Anthony KRN (1999) Coral suspension feeding on fine particulate matter. J Exp Mar Biol Ecol 232:85-106
- Anthony KRN (2000) Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19:59-67
- Anthony KRN, Fabricius K (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol 252:221-253
- Barneah O, Brickner I, Hooge M, Weis VM, LaJeunesse TC, Benayahu Y (2007) Three party symbiosis: acoelomorph worms, corals and unicellular algal symbionts in Eilat (Red Sea). Mar Biol 151:1215-1223
- Bo M (2009) Antipatharians – part two: ecology. Coralscience.org, www.coralscience.org
- Carl M (2008) Predators and pests of captive corals, 31-36. In: Leewis RJ, Janse M (Eds) Advances in Coral Husbandry in Public Aquariums – Public Aquarium Husbandry Series, Volume 2, Burgers’ Zoo, Arnhem, The Netherlands. 444 p
- Dai C-F, Lin M-C (1993) The effects of flow on feeding of three gorgonians from southern Taiwan. J Exp Mar Biol Ecol 173:57-69
- Davies AJ, Duineveld GCA, Lavaleye MSS, Bergman MJN, Van Haren H et al. (2009) Downwelling and deep-water bottom currents as food supply mechanisms to the cold-water coral Lophelia pertusa (Scleractinia) at the Mingulay Reef Complex. Limnol Oceanogr 54:620-629
- Elyakova LA, Shevchenko NM, Avaeva SM (1981) A comparative study of carbohydrase activities in marine invertebrates, Comp. Biochem Physiol 69B:905-908
- Erftemeijer PLA, Riegl B, Hoeksema BW, Todd PA (2012) Environmental impacts of dredging and other sediment disturbances on corals: A review. Mar Poll Bull 64:1737-1765
- Fabricius KE, Alderslade P (2001) Soft corals and sea fans – A comprehensive guide to the tropical shallow water genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science, Townsville, Australia. 264 p
- Fabricius KE, Benayahu Y, Genin A (1995b) Herbivory in asymbiotic soft corals. Science 268:90-92
- Fabricius KE, Genin A, Benayahu Y (1995a) Flow-dependent herbivory and growth in zooxanthellae-free soft corals. Limnol Oceanogr 40:1290-1301
- Ferrier-Pagès C, Allemand D, Gattuso JP, Jaubert J, Rassoulzadegan F (1998a) Microheterotrophy in the zooxanthellate coral Stylophora pistillata: Effects of light and ciliate density. Limnol Oceanogr 43:1639-1648
- Ferrier-Pagès C, Gattuso JP, Cauwet G, Jaubert J, Allemand D (1998b) Release of dissolved organic carbon and nitrogen by the zooxanthellate coral Galaxea fascicularis. Mar Ecol Prog Ser 172:265-274
- Ferrier-Pagès C, Hoogenboom M, Houlbrèque F (2011) The role of plankton in coral trophodynamics, 215-229. In: Dubinsky Z, Stambler N (Eds), Coral reefs: an ecosystem in transition. Springer, Dordrecht, The Netherlands
- Ferrier-Pagès C, Witting J, Tambutté E, Sebens KP (2003) Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs 22:229-240
- Fine M, Loya Y (2002) Endolithic algae: An alternative source of photoassimilates during coral bleaching. Proc R Soc B 269:1205-1210
- Goreau TF, Goreau NI, Yonge CM (1971) Reef corals: autotrophs or heterotrophs? Biological Bulletin 141:247-260
- Grover R, Maguer JF, Allemand D, Ferrier-Pagès C (2006) Urea uptake by the scleractinian coral Stylophora pistillata. J Exp Mar Biol Ecol 332:216-225
- Grover R, Maguer JF, Allemand D, Ferrier-Pagès C (2008) Uptake of dissolved free amino acids (DFAA) by the scleractinian coral Stylophora pistillata. J Exp Biol 211:860-865
- Helmuth B, Sebens K (1993) The influence of colony morphology and orientation to flow on particle capture by the scleractinian coral Agaricia agaricites (Linnaeus). J Exp Mar Biol Ecol 165:251-278
- Hoeksema BW, Farenzena ZT (2012) Tissue loss in corals infested by acoelomorph flatworms (Waminoa sp.). Coral Reefs 31:869
- Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev Camb Philos 84:1-17
- Houlbrèque F, Tambutté E, Richard C, Ferrier-Pagès C (2004) Importance of a micro-diet for scleractinian corals. Mar Ecol Prog Ser 282:151-160
- Hunter T (1989) Suspension feeding in oscillating flow: the effect of colony morphology and flow regime on plankton capture by the hydroid Obelia longissima. Biol Bull 176:41-49
- Jimenez IM, Kühl M, Larkum AWD, Ralph PJ (2011) Effects of flow and morphology on the thermal boundary layer of corals. J R Soc Interface 8:1785-1795
- Lai S, Gillis LG, Mueller C, Bouma TJ, Guest JR, Last KS, Ziegler AD, Todd PA (2013) First experimental evidence of corals feeding on seagrass matter. Coral Reefs DOI 10.1007/s00338-013-1062-9
- Leal MC, Ferrier-Pagès C, Calado R, Thompson ME, Frischer ME, Nejstgaard JC (2013) Coral feeding on microalgae assessed with molecular trophic markers. Mol Ecol doi: 10.1111/mec.12486
- Lesser MP, Falcón LI, Rodríguez-Román A, Enríquez S, Hoegh-Guldberg O, Iglesias-Prieto R (2007) Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar Ecol Prog Ser 346:143-152
- Lewis JB (2006) Biology and ecology of the hydrocoral Millepora on coral reefs. Adv Mar Biol 50:1-55
- Lin MC, Liao CM, Dai CF (2002) Modeling the effects of satiation on the feeding rate of a colonial suspension feeder, Acanthogorgia vegae, in a circulating system under lab conditions. Zool Stud 41:355-365
- Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals, 755-87. In: Dubinsky Z (Ed), Coral reefs: ecosystems of the world 25. Elsevier, Amsterdam, The Netherlands
- Naumann MS, Mayr C, Struck U, Wild C (2010) Coral mucus stable isotope composition and labeling: experimental evidence for mucus uptake by epizoic acoelomorph worms. Mar Biol 157:2521-2531
- Nosratpour F (2008) Observations of a polyclad flatworm affecting acroporid corals in captivity. In: Leewis RJ, Janse M (Eds) Advances in Coral Husbandry in Public Aquariums – Public Aquarium Husbandry Series, Volume 2, Burgers’ Zoo, Arnhem, 37-46
- Osinga R, Schutter M, Griffioen B, Wijffels RH, Verreth JAJ, Shafir S, Henard S, Taruffi M, Gili C, Lavorano S (2011) The biology and economics of coral growth. Mar Biotechnol 13:658-671
- Osinga R, Schutter M, Wijgerde T, Rinkevich B, Shafir S, Shpigel M, Luna GM, Danovaro R, Bongiorni L, Deutsch A, Kuecken M, Hiddinga B, Janse M, McLeod A, Gili C, Lavorano S, Henard S, Barthelemy D, Westhoff G, Baylina N, Santos E, Weissenbacher A, Kuba M, Jones R, Leewis R, Petersen D, Laterveer M (2012) The CORALZOO project: a synopsis of four years of public aquarium science. Journal Mar Biol Assoc UK 92:753-768
- Picciano M, Ferrier-Pagès C (2007) Ingestion of pico- and nanoplankton by the Mediterranean red coral Corallium rubrum. Mar Biol 150:773-782
- Ribes M, Coma R, Gili J-M (1999) Heterogeneous feeding in benthic suspension feeders: the natural diet and grazing rate of the temperate gorgonian Paramuricea clavata (Cnidaria: Octocorallia) over a year cycle. Mar Ecol Prog Ser 183:125-137
- Roff G, Dove SG, Dunn SR (2009) Mesenterial filaments make a clean sweep of substrates for coral growth. Coral Reefs 28:79
- Schutter M, Crocker J, Paijmans A, Janse M, Osinga R, Verreth AJ, Wijffels RH (2010) The effect of different flow regimes on the growth and metabolic rates of the scleractinian coral Galaxea fascicularis. Coral Reefs 29:737-748
- Schutter M, Kranenbarg S, Wijffels RH, Verreth JAJ, Osinga R (2011) Modification of light utilization for skeletal growth by water flow in the scleractinian coral Galaxea fascicularis. Mar Biol 158:769-777
- Sebens KP, Grace SP, Helmuth B, Maney Jr EJ, Miles JS (1998) Water flow and prey capture by three scleractinian corals, Madracis mirabilis, Montastrea cavernosa and Porites porites, in a field enclosure. Mar Biol 131: 347-360
- Swanson R, Hoegh-Guldberg O (1998) Amino acid synthesis in the symbiotic sea anemone Aiptasia pulchella. Mar Biol 131:83-93
- Tsounis G, Rossi S, Laudien J, Bramanti L, Fernández N, Gili J-M, Arntz W (2006) Diet and seasonal prey capture rates in the Mediterranean red coral (Corallium rubrum L.). Mar Biol 149:313-325
- Wijgerde T (2012a) Improved husbandry of marine invertebrates using an innovative filtration technology – part one: DyMiCo. Advanced Aquarist 11(2)
- Wijgerde T (2012b) Improved husbandry of marine invertebrates using an innovative filtration technology – part two: results with two 12 m3 DyMiCo systems. Advanced Aquarist 11(3)
- Wijgerde T (2013a) Zooxanthellae: Biology and Isolation for Scientific Study. Advanced Aquarist 12(5)
- Wijgerde T (2013b) Heterotrophic feeding, growth and nutrient budget in the scleractinian coral Galaxea fascicularis. PhD thesis, Wageningen University, Wageningen, The Netherlands
- Wijgerde T, Diantari R, Lewaru MW, Verreth JAJ, Osinga R (2011a) Extracoelenteric zooplankton feeding is a key mechanism of nutrient acquisition for the scleractinian coral Galaxea fascicularis. J Exp Biol 214: 3351-3357
- Wijgerde T, Henkemans P, Osinga R (2012a) Effects of irradiance and light spectrum on growth of the scleractinian coral Galaxea fascicularis – Applicability of LEP and LED lighting to coral aquaculture. Aquaculture 344-349:188-193
- Wijgerde T, Jurriaans S, Hoofd M, Verreth JAJ, Osinga R (2012b) Oxygen and heterotrophy affect calcification of the scleractinian coral Galaxea fascicularis. PLoS ONE 7(12): e52702. doi:10.1371/journal.pone.0052702
- Wijgerde T, Schots P, van Onselen E, Janse M, Karruppannan E, Verreth JAJ, Osinga R (2012c) Epizoic acoelomorph flatworms impair zooplankton feeding by the scleractinian coral Galaxea fascicularis. Biol Open 2:10-17
- Wijgerde T, Spijkers P, Karruppannan E, Verreth JAJ, Osinga R (2012d) Water flow affects zooplankton feeding by the scleractinian coral Galaxea fascicularis on a polyp and colony level. J Mar Biol doi:10.1155/2012/854849
- Wijgerde T, Spijkers P, Verreth J, Osinga R (2011b) Epizoic acoelomorph flatworms compete with their coral host for zooplankton. Coral Reefs 30:665
- Purser A, Larsson AI, Thomsen L, van Oevelen D (2010) The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. J Exp Mar Biol Ecol 395:55–62










0 Comments