
Looking up the word myth in a dictionary yields several results, including this: a fiction or half-truth, especially one that forms part of an ideology. This suggests that myths do not need debunking, otherwise they would be called facts. The problem is that myths are often presented as facts. Although Jury (2006) did a wonderful job debunking some of the aquarium myths, or “facts” described below, it seems that some of them are quite persistent. This has led us to the present article, in which we scrutinize these myths from a scientific perspective.
“The more light corals receive, the better”
This is probably one of the most persistent myths in the marine aquarium hobby today, and to be honest, it is partially true. Based on the belief that more light is always better, many aquaria are equipped with as much lighting power as people can afford. This myth stems from the notion that shallow-growing corals are exposed to very high light intensities. Indeed, corals growing in intertidal zones are exposed to air several times a day during low tide, blasted by the full power of the sun, with light intensities exceeding 2,000 µmol m-2 s-1 as photosynthetically active radiation (PAR, ~400-700 nm) (Huang et al. 1995; Demmig-Adams et al. 1996). For comparison, most aquaria receive an irradiance in the range of 100-400 µmol m-2 s-1 (Kirda 2003). Although it is true that corals can be exposed to very high light intensities, this is not always beneficial to the corals. This is because their symbiotic dinoflagellates, or zooxanthellae, receive so much light that it saturates and even damages their photosynthetic machinery (Osinga et al. 2011).
To cope with this huge excess of (UV) light, both the corals and zooxanthellae use various mechanisms to prevent massive tissue damage. One of the mechanisms used by the coral is the production of mycosporin-like amino acids (MAA’s) and fluorescent proteins (Kinzie 1993; Dunlap and Shick 1998; Salih et al. 2000). MAA’s shield the coral and its zooxanthellae from harmful UV radiation that would otherwise cause major damage to cell membranes and DNA. Fluorescent proteins, such as the attractive green fluorescent protein (GFP) which lights up under blue light, is thought to protect the coral against high levels of oxygen radicals produced via photosynthesis (Salih et al. 2000; Bou-Abdallah et al. 2006). Cyan fluorescent proteins may reduce some of the light that zooxanthellae receive for photosynthesis (D’Angelo et al. 2008). Another strategy used by corals is polyp retraction, shading the zooxanthellae from excess light (dimly lit corals usually show increased polyp extension). In addition, the coral skeleton is able to fluoresce UV radiation as yellow light, possibly reducing tissue damage (Reef et al. 2009). The zooxanthellae, in turn, dissipate excess light energy as heat via a complex process known as non-photochemical quenching, and constantly repair their photosynthetic machinery to keep it working (Warner et al. 1999; Jones et al. 2001; Hill et al. 2005).
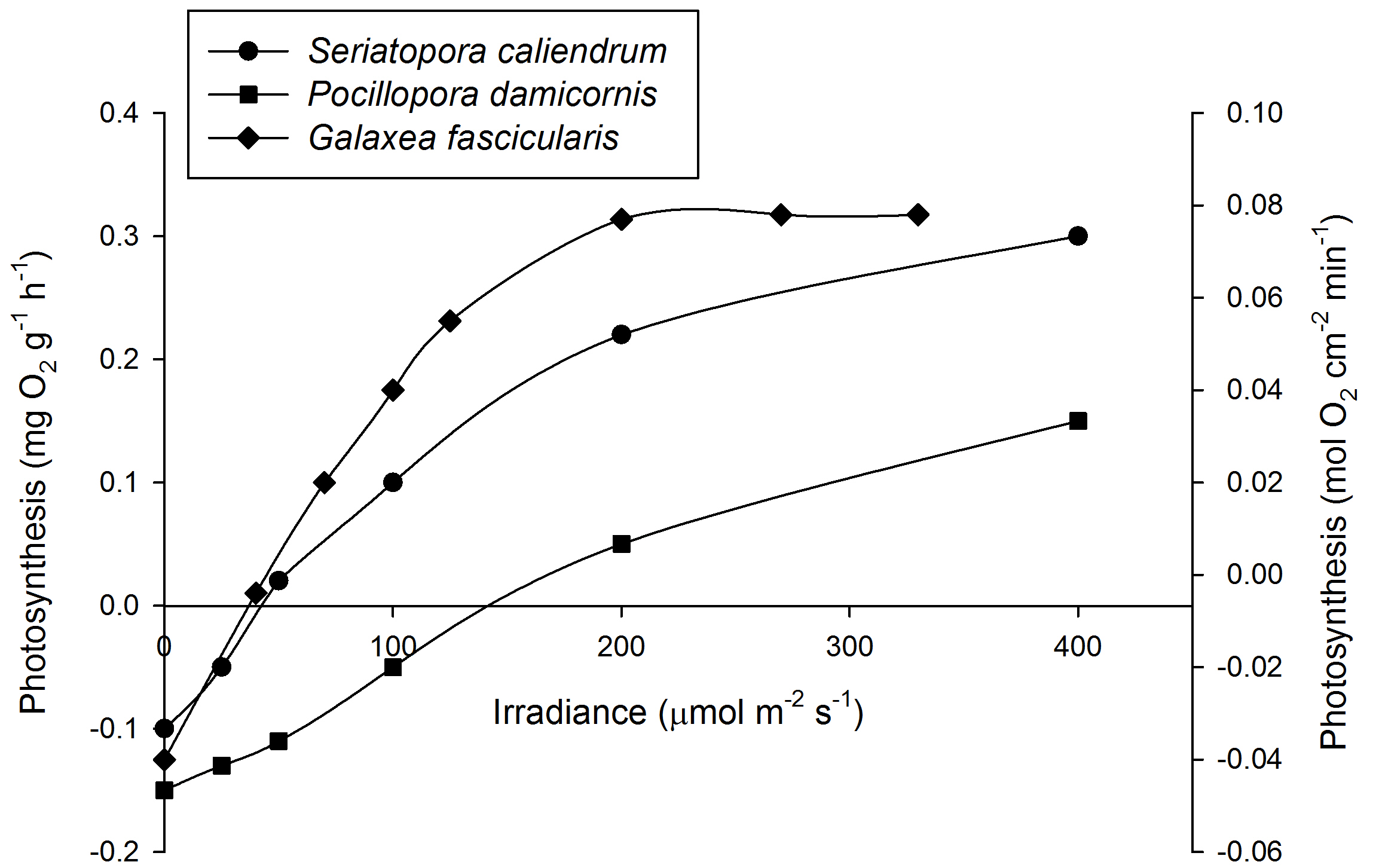
Mean Photosynthesis rates of Seriatopora caliendrum (left axis, N=3), Pocillopora damicornis (left axis, N=3) and Galaxea fascicularis (right axis, N=4) under various irradiance levels.At 200 µmol m-2 s-1, photosynthesis has saturated for G. fascicularis. For clarity, error bars have been omitted. Based on Osinga et al. (2011).
Thus, corals do not require the excessive light intensity found in shallow water, but rather have to cope with it, and thrive at lower irradiances. In fact, zooxanthellate stony corals have been found down to a depth of 167 meters, and are commonly found below 100 meters (333 feet) in depth (Rooney et al. 2010; Bongaerts et al. 2011). Here, corals may receive less than 0.1% of the sunlight present at sea level. To compensate for this, the zooxanthellae produce more photopigments such as chlorophylls to efficiently harness the feeble light that they receive. In addition, the coral skeleton acts as an efficient light collector, scattering light in such a way that the zooxanthellae can effectively use it (Enriquez et al. 2005). Corals are so sensitive to visible light, that they can detect it at an irradiance of merely 0.002 µmol m-2 s-1, allowing them to sense sunlight at great depth, and faint moonlight in shallow water (Gorbunov et al. 2002). Finally, deep-growing corals may feed on plankton, detritus and dissolved matter to compensate for the decreased amount of nutrients obtained through photosynthesis (Anthony and Fabricius 2000; Titlyanov et al. 2000, 2001a). Additional nitrogen obtained via feeding also allows zooxanthellae to persist at very low light levels (Titlyanov et al. 2000, 2001a).

Specific growth rates of Acropora millepora (N=18) and Montipora aequituberculata (N=18) under various irradiance levels. Growth interval was 70 days. Increasing irradiance beyond 50 µmol m-2 s-1 has a saturating effect on A. millepora, and a growth-inhibiting effect on M. aequituberculata (graph: T. Wijgerde, based on Wijgerde and Laterveer 2013).
After acclimation, an irradiance of 200-300 µmol m-2 s-1 is sufficient to saturate photosynthesis in several coral species such as Galaxea fascicularis (Riddle 2007; Schutter et al. 2008, 2011), although some species require more light. For example, Seriatopora caliendrum and Pocillopora damicornis require over 400 µmol m-2 s-1 to saturate photosynthesis. It must be noted, however, that even these corals may saturate at lower levels when acclimatized to low light intensities. In addition, each genetically unique individual within a species may behave differently. In terms of growth, Montipora aequituberculata already shows a growth saturation at 40-60 µmol m-2 s-1, although other species such as Acropora millepora saturate at higher light levels of 300 µmol m-2 s-1 and beyond (Wijgerde and Laterveer 2013).
So, to be fair, this first myth is partially true. More light can indeed be better for some corals, in terms of photosynthesis and growth, but only up to a certain point. The same goes for inducing coral coloration, as high light intensities are required for the production of several green and red fluorescent proteins, and non-fluorescent chromoproteins (D’Angelo et al. 2008; Wijgerde and Laterveer 2013). However, at higher irradiance, corals show less efficient relative growth (Schutter et al. 2008; Wijgerde and Laterveer 2013), and can become stressed. This is especially true when water flow rate is too low, possibly preventing the coral from releasing excess heat and oxygen produced through photosynthesis (Finelli et al. 2006, 2007; Fabricius et al. 2006; Mass et al. 2010; Jimenez et al. 2011). Thus, we can debunk another, related myth: highly colorful corals are not necessarily the happiest corals, but actually stressed, and have to invest a significant amount of energy into protective mechanisms (including the production of colorful pigments) to prevent light-induced damage.
“Stony corals require more light than soft corals”

A Sarcophyton and Acropora colony growing alongside one another at a reef bommie in the Red Sea, Ra’s Qul`an, Egypt. Approximate depth is 3 meters, or 10 feet (image: T. Wijgerde).
This myth is clearly linked to the first one, and probably just as persistent. A common phrase, or some version thereof, is: “If you only have soft corals, 100 Watts of lighting power is enough, but if you want to keep stony corals, you will need at least twice that.” When we take a dive on coral reefs, we actually find that the view that stony corals require more light than soft corals has no factual basis. On very shallow reefs, we find both soft and hard corals, growing alongside one another. We see the same when we go deeper, even below 100 meters (333 feet), where both soft and hard corals such as Sarcophyton, Lobophyton, Acropora and Leptoseris spp. are found (Riddle 2007; Bare et al. 2010; Rooney et al. 2010; Bongaerts et al. 2011). These observations are in agreement with laboratory experiments, revealing that soft corals sometimes require more light than stony corals to saturate their photosynthesis rates (Riddle 2007).
The question is why this myth has been able to pervade the hobby. Perhaps it stems from the changes in coral pigmentation when light levels decrease. A common phenomenon many aquarists have observed is an attractively colored Acropora colony turning brown within one or two weeks after being introduced into the home aquarium. This is caused by the lower availability of light (and sometimes the higher availability of inorganic nutrients, see below), coaxing the zooxanthellae into producing more chlorophylls and other photopigments within a time frame of several days, making the coral appear brown (Titlyanov et al. 2000, 2001a,b). Sometimes, the density of zooxanthellae also increases, depending on the species, a process which takes several weeks (Kinzie et al. 1984; Titlyanov et al. 2000, 2001a,b). Thus, the coral and its zooxanthellae are simply adapting to the decreased availability of light in the aquarium, by making the photosynthetic machinery more efficient. As brown is a color most people find less attractive than purple, green, blue, yellow or red, the coral may appear stressed or ill, causing the observing aquarist to believe that the Acropora in question needs more light (while in fact, it seems that the aquarist him/herself needs more). This example shows how adaptive corals really are, and how our perception of what is right can cause us to misunderstand these unique animals.
“Aquarium lights that appear blue are more natural and better for corals”

Many corals such as this Montipora colony have green tissues that fluoresce intensely under blue and violet light, making actinic light sources highly popular (image: T. Wijgerde).
Although many different lighting types are used above marine aquaria nowadays, most of them have something in common: they emit a significant amount of blue light. Most hobbyists prefer their marine aquarium to be bathed in blue light, bringing out the fluorescent properties of their invertebrates, whilst their planted freshwater tank receives yellowish light. This blue/yellow dichotomy between marine and fresh water aquaria is believed to be natural, and useful for corals and marine fish. While blue light is certainly aesthetically pleasing, a light source that is skewed toward the blue part of the spectrum is not per se natural, or essential to corals. If we look at how seawater affects the spectral composition of sunlight, we can say that between 0 and approximately 10 meters (33 feet), all colors within the visible light spectrum are present. After 10 meters, however, the amount of red light quickly decreases, leaving the blue-green hue so familiar to divers. This means that even “yellow” light sources, which emit blue, red and other colors, can be both natural and well-suited for a coral aquarium. It all depends on what part of the water column we would like to recreate. While it is true that some corals prefer blue light, and others seem to benefit from a more balanced light source, in terms of growth rate (Wijgerde and Laterveer 2013), most corals do well under a variety of light spectra.
“The more water flow corals receive, the better”

Plerogyra sinuosa has polyps with large, fleshy tentacles that easily tear when exposed to strong, direct flow (image: T. Wijgerde).
Again, in all fairness, this myth is partially true. Providing corals with more water flow has beneficial effects on growth rates, photosynthesis, respiration, sediment shedding, waste removal and feeding (Wijgerde 2013b). However, “too much” is a concept that also holds true for water flow, as many aquarists know well. For example, placing corals too close to the outlet of a flow pump can result in serious damage to coral tissue. In addition, when corals experience very high flow rates, their polyps deform to such an extent that feeding on plankton is no longer possible (Dai and Lin 1993; Wijgerde et al. 2012b).
In practice, most corals do well and are able to feed in a flow range between roughly 5 and 25 cm s-1. Examples of corals that are quite selective in terms of flow rate are the gorgonians Acanthogorgia vegae, Melithaea ochracea and Subergorgia suberosa (Dai and Lin 1993), the azooxanthellate stony coral Lophelia pertusa (Purser et al. 2010) and octocorals such as Dendronephthya spp. (Fabricius et al. 1995). For details on optimal flow regimes for these species, see Wijgerde (2013a).
“Dosing trace elements is essential to maintain healthy and colorful corals”
This myth seems to be supported by only a subset of aquarium hobbyists. Many additives for marine aquaria are on the market today, which include macro elements (e.g. calcium, magnesium, potassium), pH and alkalinity boosters, trace elements and organic supplements (e.g. amino acids). Although the science behind some of these additives is valid, it is not always necessary to dose supplements to the aquarium in order to keep healthy and colorful corals. In our coral lab at Wageningen University, some aquaria have been running for about two years, with healthy corals, without any trace element additives. These aquaria received 10% weekly water changes, and calcium carbonate and sodium bicarbonate to maintain calcium and alkalinity levels. The water changes served to maintain relatively stable concentrations of magnesium, potassium, and various trace elements. Regular feeding with Artemia nauplii provided the corals with proteins and other organic compounds. This works for most aquaria because many commercially available sea salts are rich in trace elements, resulting in artificial seawater with many trace elements (greatly) exceeding natural levels.

Concentrated trace elements are sometimes used to promote coral coloration, at the risk of aquarium life (image: T. Wijgerde).
Of course, some aquaria may be so heavily stocked with corals that the demand for trace elements is accordingly high that frequent water changes are insufficient. In that case, dosing concentrated additives is recommended, but this diagnosis requires meticulous water analysis in a specialized laboratory. Dosing highly concentrated trace elements without prior testing to determine whether it is required or not can be harmful to corals and other invertebrates. Heavy metals and halogens such as iodine and fluorine, on occasion added to promote the coloration of stony corals, are toxic when present in unnaturally high concentrations. Although experienced aquarists claim they can determine whether specific elements are depleted by looking at the shape, color and polyp extension of their corals, it is prudent to get objective results before anything is added to the aquarium.
A good example is an experimental coral nursery in The Netherlands, where about 3,000 corals were cultured in two 12,000 liter (3,158 US gallon) basins. After one year of operation, without water changes, one might have thought that certain additives would be required to maintain coral growth and coloration. However, after laboratory analysis, many elements were found to exceed natural levels (following Spotte 1992), which included potassium, barium, cadmium, cobalt, chromium, copper, iron, lanthanum, lead, antimony and tin (Wijgerde 2012). Similar observations were made in our lab at Wageningen University, although different aquaria had different elements with unnatural levels. Although the aquarium industry promotes the dosing of trace elements to home aquaria, it is clear that without the proper analytical methods, it is impossible to determine what element(s) require adjusting. Although bacteria and algae may sequester some of the surplus in trace elements from the seawater (Martin and Knauer 1973), it is likely that many home aquarists are currently overdosing a range of toxic elements. Good methods to determine the elemental composition of aquarium water are ICP-AES and ICP-MS, which allow for accurate analysis of macro and trace elements, respectively.
“Corals cannot grow, and usually die, when nitrate and phosphate levels are high”
Nutrient levels in aquaria are a topic of much interest and discussion, with conflicting opinions. Three reasons why aquarists may vote against maintaining high nutrient levels in aquaria are corals turning brown, increased growth of nuisance algae, and the notion that nutrient levels on natural reefs are very low. When evaluating what happens to corals when they are exposed to elevated nitrogen or phosphorus concentrations, we have to make a clear distinction between the primary physiological effects, and the secondary ecological effects.

Not the most ideal environment one might think; an aquarium full of phosphate (around 2 mg L-1) and Aiptasia. Still, these corals grew well, although the lack of water flow caused some damage to the inner parts of the colonies (image: T. Wijgerde).
To study eutrophication, several studies have elevated the nitrogen concentration to 20 µmol L-1 (equal to 1.24 mg L-1 nitrate). For phosphorous, this elevation often is a concentration of 2-3 µmol L-1 (or up to 0.28 mg L-1 orthophosphate). This increase in nutrient concentrations has been found to have a sublethal, but growth-inhibiting effect on many coral species (Marubini and Davies 1996; Ferrier-Pagès et al. 2000; Tanaka et al. 2007; Hylkema et al. 2014), which is caused by changes in the coral’s physiology. Several theories exist that help explain why eutrophication has a negative effect on coral growth. These include crystal poisoning, where polyphosphate crystals negatively affect coral growth by preventing new skeleton from being formed (Dunn et al. 2012), increased competition between corals and zooxanthellae for bicarbonate and CO2 (Allemand et al. 2004), and reduced release of photosynthates to the corals by zooxanthellae (Falkowski et al. 1993; Stambler 2011). The theory of competition between corals and zooxanthellae for bicarbonate and CO2 is plausible, as zooxanthellae density increases at elevated inorganic nutrient concentrations, requiring more substrate for photosynthesis (Muscatine et al. 1989; Dubinsky et al. 1990; Stambler et al. 1991; Falkowski et al. 1993; Marubini and Davies 1996; Titlyanov et al. 2000). Indeed, bicarbonate supplementation prevents coral growth from being negatively affected by eutrophication (Marubini and Thake 1999).
Next to the direct physiological effects, the secondary, ecological effects of eutrophication include overgrowth of corals by algae, increased sedimentation and decreased light availability due to plankton blooms, and mortality of reef fauna due to oxygen depletion (Guzmán et al. 1990; Fabricius et al. 2005; Mumby and Steneck 2008; Bauman et al. 2010). When sufficient herbivores are present, and adequate filtration is used, elevated N and P levels are not necessarily detrimental, as many aquarists have found out. Although it is true that elevated nitrate and phosphate levels can negatively affect coral growth, change coral coloration, and increase the growth of algae and cyanobacteria, it does not seem to directly cause coral mortality.
“LPS and SPS are meaningful terms for grouping stony corals”
In an effort to find some definitions for the terms SPS and LPS, we mainly came across definitions that only describe the full length of the acronyms. LPS is short for Large Polyp(ed) Stony corals, and SPS stands for Small Polyp(ed) Stony corals. LPS corals are “known” as having thicker, fleshier tissue compared to SPS corals. LPS corals are also stereotyped as easier to keep than SPS corals. The terms are thought to originate from the 1980’s when certain types of corals (LPS) would survive longer, or died more slowly than corals such as Acropora and Montipora spp. (SPS). While we can agree that LPS and SPS can be used to create a dichotomy between corals, the terms lack a properly quantified definition to hold any merit in scientific writing. Both “small” and “large” are subjective terms. In an interview, an experienced aquarist qualified large as anything with a polyp diameter larger than 4 cm (or ~1.5 inches). According to that definition, most genera currently assigned to LPS would no longer be LPS (e.g. Blastomussa, Acanthastrea, Caulastraea), leaving genera such as Fungia, Scolymia and Cynarina. Thus, even a specific size is not sufficient for a good definition as it is highly arbitrary. Studying the detailed morphology of coral skeletons, together with DNA analysis, is the only reliable way to distinguish between species (also see below). In addition, as size is not a good predictor for the environment in which a coral species is found, this characteristic has little value when determining what a coral requires in captivity.
“Form and color are good criteria for identifying corals”

Variations in morphology of the coral Pocillopora verrucosa under several flow regimes. New branches form on the upstream side of the flow, and get much denser as flow rate increases from 1 to 5 cm s-1. Arrows indicate flow direction. Modified from Chindapol et al. 2013.
Next to the LPS and SPS myth, a popular belief is that growth form and coloration are reliable criteria for identifying and discriminating between coral species. Unfortunately, this again is misleading. This is because the appearance, or phenotype, of corals depends on several factors including light and water flow. Corals are plastic in nature, which means they can change in appearance, to a certain degree, in response to changes in the environment. A nice example of this so-called phenotypic plasticity is the coral Pocillopora damicornis. This species can be found in many environments, resulting in a variety of morphotypes (Veron 1995; Schmidt-Roach et al. 2014). On reef crests, P. damicornis forms thick branches, possibly to cope with the prevailing high water flow. On deeper parts of the reef, but also in lagoons, this coral produces much thinner, delicate branches.

Zoanthids (order Zoanthidea/Zoantharia, subclass Hexacorallia) are polyps that exist in many different color patterns, and DNA analysis is required to properly identify them (image: T. Wijgerde).
It is also clear that corals from the genus Pocillopora mainly form new branches on the side where water flow is present, resulting in asymmetrical growth when colonies are exposed to unidirectional flow (Chindapol et al. 2013). Thus, the form of corals is not only determined by species (each with its own genetic programming), but also by the prevailing environmental conditions. As mentioned above, coral color can also vary depending on light and water quality, making this attribute a poor choice for coral taxonomy as well. In addition, individuals (i.e. different genotypes) within a coral species can have many different color morphs.
To make matters worse, corals are also known to hybridize, sometimes yielding fertile offspring (Marti-Puig et al. 2014). So how do we identify and classify corals? Species identification was, for many years, based on morphological aspects of the coral skeleton, such as the pattern and structure of septa and columella (Richmond and Wolanski 2011). The emergence of molecular genetics has improved our understanding of coral taxonomy and has changed the way we classify corals. Recently, many corals have been taxonomically revised based on genetic evidence (Budd et al. 2012). For example, the genus Galaxea, a former member of the Oculinidae family, has been moved to the Euphylliidae. Another striking example is Trachyphyllia geoffroyi, the only former species within the Trachyphylliidae, which is now included in the Merulinidae family. Still, the micromorphology of corals, together with molecular genetics, will continue to play a role in species identification (Marti-Puig et al. 2014).
“Popular names are more practical than scientific ones”
Next to using the wrong criteria for identifying corals, the use of popular names for corals and fish has been a source of confusion. Often people only use colloquial names to describe their aquarium inhabitants, thinking it is more practical. However, these colloquial names are only applicable to one specific language, and oftentimes, many synonyms exist. The major issue, however, is that these popular names lack a useful structure, and thus give no indication of the relationship between organisms. For this reason, we urge people to always use the scientific nomenclature, rather than dubious names such as Candy Cane Coral, Bird’s Nest Coral or Spider Sponge. Although coral taxonomy currently is in a state of flux due to new genetic insights, making it more difficult to remember scientific names, it still is more practical than using countless (international) synonyms. Proper use of scientific names is as follows: Genus species, e.g. Acropora millepora (not to be confused with the hydrocoral genus Millepora). The entire name should be written in cursive, with the genus name starting with an upper case and the species name with a lower case. When referring to one or more species within a genus, you can use the abbreviations “sp.” (one species) or “spp.” (multiple species), both of which are not written in cursive. Because scientific names regularly change, we often mention the person and the year in which the name was given, e.g. Stylophora pistillata Esper 1797, either with our without parentheses. Although these Latin names may sound difficult, they are highly practical as they tell us to what extent different corals are related.

Should we use interchangeable names such as Randall’s Shrimp Goby, Randall’s Prawn Goby and Orange Stripe Prawn Goby, or simply choose one, Amblyeleotris randalli (image: T. Wijgerde)?
We can extend the use of scientific names to higher taxonomic levels, to understand more about the relatedness of the thousands of coral species that exist today. Taxonomy refers to the classification system of organisms, which originated in the 18th century by virtue of Carolus Linnaeus (or Carl von Linné) and which is still used today. It consists of multiple levels of organization, where higher levels represent larger groups. As an example we can use Acropora millepora again:
- Domain: Eukaryota (nucleated cells with organelles) Whittaker and Margulis 1978
- Kingdom: Animalia (animals) Linnaeus 1758
- Phylum: Cnidaria (animals with cnidocytes, or stinging cells) Hatschek 1888
- Class: Anthozoa (flower-like animals) Ehrenberg 1834
- Subclass: Hexacorallia (corals/anemones/polyps with six-fold symmetry) Haeckel 1896
- Order: Scleractinia (hard anemone-like corals) Bourne 1905
- Family: Acroporidae (Acropora-like corals) Verrill 1902
- Genus: Acropora Oken 1815
- Species: millepora Ehrenberg 1834
Humans (Homo sapiens), for instance, belong to the same kingdom as Acropora millepora; true jellyfish (class Scyphozoa) belong to the same phylum; anemones (order Actiniaria) and soft corals (order Alcyonacea) belong to the same class; black corals (order Antipatharia) belong to the same subclass; Fungia corals (family Fungiidae) belong to the same order; Montipora spp. belong to the same family; and finally, the species Acropora humilis belongs to the same genus. According to this system, it would for example be incorrect to state that the genera Fungia and Acropora belong to the same family, as they belong to the same order only. If the term “group” is used, one can never be wrong because two given coral species will always meet somewhere in the taxonomic tree, although this term is rather vague.
“Planaria are found in marine aquaria”
Perhaps this is not exactly a myth, but just an erroneous use of a very common term. Planaria has become a colloquial term for describing flatworms in marine aquaria, which is probably based on the family Planariidae (order Tricladida), and/or the genus Planaria. Interestingly, all members of the Planariidae family inhabit fresh water. Most flatworms that are found in marine aquaria are those that usually dwell on corals, and these are either acoelomorph or polyclad flatworms. It is therefore our opinion that the term flatworms has preference over the misleading term planaria.
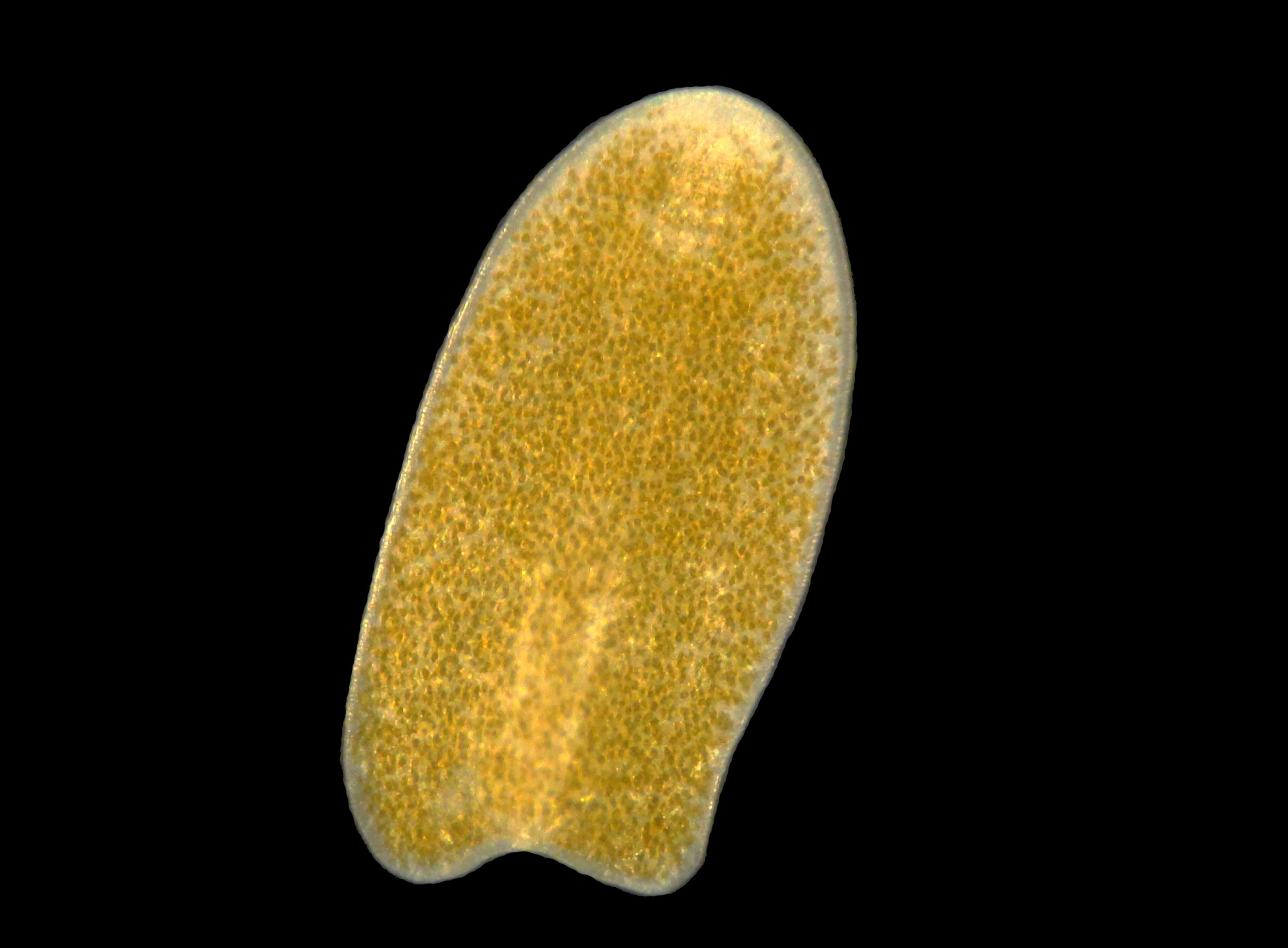
An acoelomorph flatworm, most likely a Waminoa sp., found in an aquarium. Note the abundant zooxanthellae in the flatworm, possibly Symbiodinium or Amphidinium sp. (image: T. Wijgerde).
Acoelomorph flatworms lack a body cavity, and digest their prey in a so-called syncytium, a mass of interconnected cells. In aquaria, we find members of the family Convolutidae, within the disputed order Acoela and phylum Acoelomorpha (WoRMS 2013). Species that are regularly found on corals are from the genera Waminoa and Convolutriloba, and they can be distinguished from one another by looking at the posterior end of the worms. This has either two or three lobes, placing the worms in the genus Waminoa and Convolutriloba, respectively. Further identification at species level requires the use of DNA sequencing. Waminoa and Convolutriloba spp. are known to prey on zooplankton (Hendelberg and Åkesson 1988; Shannon and Achatz 2007; Wijgerde et al. 2011), and at least Waminoa spp. show parasitic behaviour, impairing coral feeding and stealing prey acquired by the host coral (Wijgerde et al. 2012a). Acoelomorph flatworms may be kept under control by introducing natural predators such as wrasses (e.g. Halichoerus spp.), dragonets (e.g. Synchiropus splendidus) and nudibranchs (Chelidonura varians) (Carl 2008; Nosratpour 2008). Chemical treatment of corals with the anthelmintic levamisole also works well, but this is impractical for whole aquaria and may negatively affect corals.
Polyclad flatworms are quite different, and are placed in the order polycladida, class Turbellaria. The most notorious polyclad flatworm is Amakusaplana acroporae (Prosthiostomidae, Rawlinson 2011), which is known to devour entire Acropora colonies (Nosratpour 2008). In contrast to acoels, which only seem to feed on coral mucus and zooplankton, A. acroporae appears to consume coral tissue. This theory is supported by the presence of nematocytes, cells used by the coral to immobilize prey, in the worm’s digestive tract (Rawlinson 2011). Polyclad flatworms may also be controlled by the predators listed above, and can be removed by spraying them with fresh water (Nosratpour 2008).
“Bleaching is the same as necrosis”
In the aquarium hobby, corals that show their white, aragonite skeleton are often diagnosed as bleached. In many instances, this diagnosis is false. Bleaching is defined as a loss of zooxanthellae and/or photopigments, necrosis is loss of coral tissue. Because bleached coral tissue is quite transparent, the skeleton below becomes visible, causing people to think the coral is dead. When necrosis occurs, corals lose their tissues, sometimes after having bleached, sometimes when the zooxanthellae population is still intact. Rapid tissue necrosis, in the hobby referred to as RTN, is an example where coral tissue sloughs off together with the zooxanthellae.
Possible causes of bleaching are high light exposure (Brown et al. 1994; Le Tissier and Brown 1996), elevated water temperatures (Lesser et al. 2007) and a high concentration of zooxanthellae (Cunning & Baker 2012). Although bleaching is highly stressful to corals, and often leads to their death, corals regularly recover from this. If a bleached coral is re-infected with new zooxanthellae, it can become viable again (Glynn and D’Croz 1990). Sometimes, this leads to a more robust colony than before. If corals are bleached due to elevated water temperatures, they can express what is known as adaptive bleaching. The stressed zooxanthellae are expelled, after which the coral takes up more thermally tolerant zooxanthellae (Baker 2001; Baker et al. 2004; Berkelmans and van Oppen 2006, Mieog et al. 2007; Jones et al. 2008). Often the coral already hosts these thermally tolerant zooxanthellae, but in a low concentration only. Without the competition of the less thermally tolerant zooxanthellae, the more tolerant ones can establish dominance. Adaptive bleaching is yet another example of phenotypic plasticity in corals, and it now is a well-known response to changing environmental conditions. However, Coffroth et al. (2010) suggest this is insufficient adaptation to deal with the effects of future climate change. Interestingly, corals still harbor several types of zooxanthellae after many years in captivity, suggesting that in the aquarium, adaptive bleaching can occur as well (Tilstra 2012; Tilstra et al. 2014).

Close-up images of Stylophora pistillata fragments during a bleaching experiment. Left: healthy coral. Middle: the same coral almost completely bleached. Right: a different coral which has completely necrotized. Notice the visible septa and empty corallites (Tilstra et al. 2014, image: S. Boele-Bos).
A direct cause of necrosis may be bleaching, where corals cannot acquire sufficient nutrients via feeding to compensate for the loss of nutrients normally gained through zooxanthellae. Various pathogens have also been implicated, including fungi and bacteria (Rosenberg and Ben-Haim 2002; Bourne et al. 2009). Luna et al. (2007) have shown that the abundance of Vibrio bacteria is higher in necrotic corals when compared to healthy corals, suggesting that bacteria play a role in this process. A distinction between bleaching and necrosis is that necrotic corals exhibit clearly visible septa and empty corallites.
One can treat bleached corals by improving whatever caused the coral to bleach in the first place. For example, corals that were bleached due to light or heat stress can recover within a few weeks when irradiance and temperature are decreased. Necrotic corals, however, should be treated differently. When a coral exhibits necrosis, the colony may be saved by removing the diseased area, preferably including a margin of unaffected tissue.
“Zooxanthellae and diatoms are plants”
This myth can confuse hobbyists and scientists alike, which is partly due to the flexible use of colloquial terms. Zooxanthellae (class Dinophyceae, or dinoflagellates) and diatoms (class Bacillariophyceae) can be described as single-celled animals with plant-like characteristics, or single-celled plants with animal-like features. When explaining the symbiosis between corals and zooxanthellae to lay audiences, scientists often refer to zooxanthellae as tiny plants. In reality, plants have their own kingdom, Plantae, and zooxanthellae and diatoms are not part of that kingdom. Neither are they bacteria. In fact, these protists are more similar to human cells as they are to bacterial cells. Bacteria lack a nucleus and belong to the domain of Prokaryota, while zooxanthellae and diatoms belong to the domain of Eukaryota. A recent change in classification assigned both dinoflagellates and diatoms to the kingdom of Chromalveolata, although they were previously known as Protista (protists). The latter term is still preferred by scientists.
Most dinoflagellates and diatoms are photosynthetic, something they have in common with true plants. Plants are mostly multicellular, while protists are found as single-celled organisms or cell colonies. In addition, protist cells have different cell walls than plants. For example, diatoms are encased in porous silica frustules, which can have all sorts of fascinating shapes. Moreover, protists are motile. Dinoflagellates have whip-like appendages called flagella (hence their name) used for “swimming”, whereas benthic diatoms have what is called a raphe, or seam, for “crawling”. Both protists are known for creating problems in marine aquaria. When conditions are right, they grow explosively and create a thick, unattractive layer coated with oxygen bubbles produced by photosynthesis. Unlike popular belief, dinoflagellates do not require silicates to grow (Tuttle and Loeblich 1975), only diatoms and some other protists do.
Dinoflagellates that are most familiar to us are zooxanthellae (genus Symbiodinium), living in symbiosis with corals, clams, nudibranchs, flatworms and foraminifera (Pawlowski et al. 2001; Venn et al. 2008). At present, nine phylogenetic clades (A to I) have been identified (Wagner et al. 2011). Each clade is divided into subclades or types. Six clades are mostly associated with reef-building or scleractinian corals (clades A to D, F and G), and clades A to D are most abundantly found (Baker 2003; Pochon 2006). Corals usually host one dominant type of zooxanthellae(Coffroth et al. 2001) and several other types in lower densities (Goulet and Coffroth 2003). There is much known variation in traits among clades and types. For example, clade D is universally known as the most thermally tolerant (Rowan 2004), while clade C1 seems to be better able to deal with low light levels compared to type C2 (Ulstrup and van Oppen 2003).
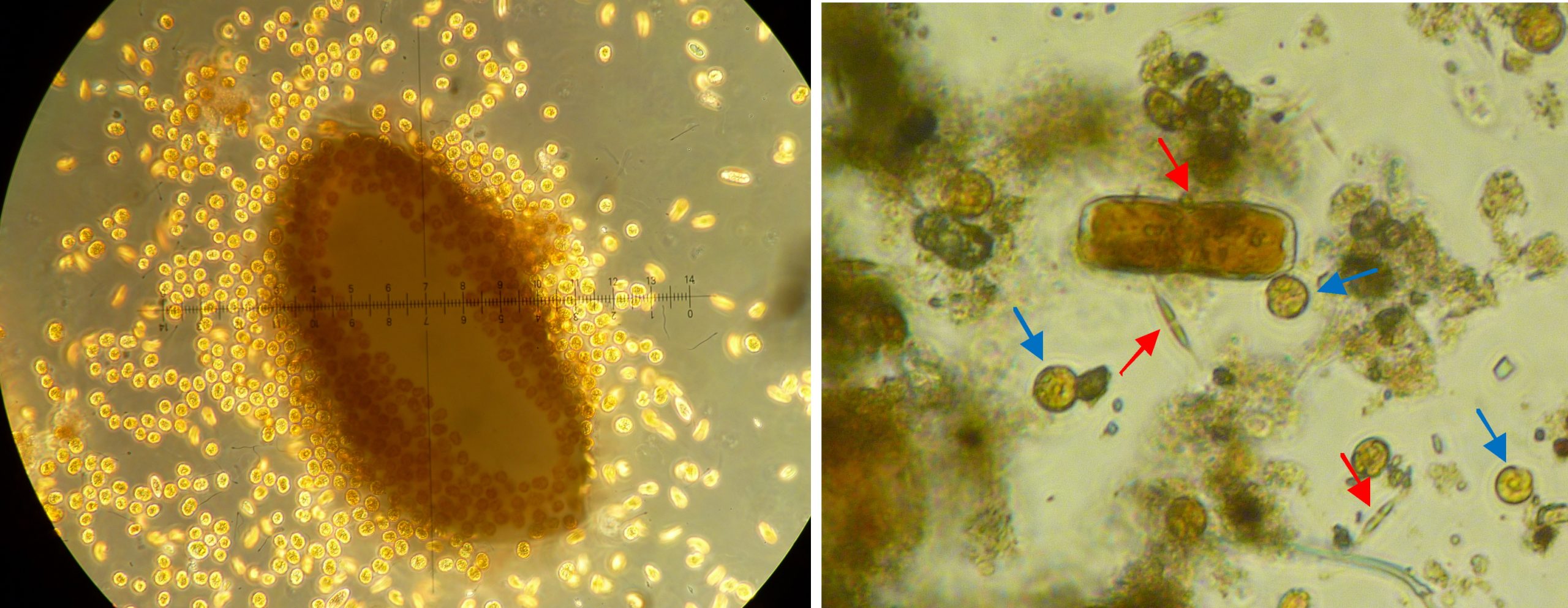
Left: a grain of coral sand completely surrounded by dinoflagellates at 100x magnification, sample taken from an algae-infested aquarium. Right: close-up of detritus found in a coral research aquarium at 100x magnification. Next to organic material, there are diatoms (red arrows) and dinoflagellates (blue arrows) (Images: A. Tilstra and P. de Vries).
Both dinoflagellates and diatoms can be part of the microphytobenthos, a layer of protists, algae and bacteria on the floor of a lake, ocean or aquarium. A microphytobenthos community is usually dominated by diatoms (Admiraal 1984; Yallop et al. 1994). Together with the associated bacteria, this community is also called periphyton. Diatoms form a microbial biofilm made of Extracellular Polymeric Substances (EPS), carbohydrate excretions which create adhesion and stability (Hoagland et al. 1993). These carbohydrate exudates are also used by the associated (heterotrophic) bacteria as a carbon-rich food source (Middelburg et al. 2000). Detritus and microphytobenthos samples taken from our research aquaria at the University of Groningen show the presence of diatoms and dinoflagellates, as well as foraminifera and sponge spicules.
“A protein skimmer is required to maintain healthy corals and fish”

This 500 liter (132 US gallons) aquarium with healthy corals was connected to a culture basin with a DyMiCo reactor installed. Save for some cyanobacteria on its back panel, this aquarium was doing just fine (image: T. Wijgerde).
It is fair to say that the protein skimmer, or foam fractionator, is an innovation that made coral husbandry as we now know it possible. Today, most marine aquaria have a protein skimmer as primary filtration, sometimes supported by fluidized, UV, sand and biofilters. Sometimes people argue that without a protein skimmer, it is impossible to maintain healthy corals and fish. While it can indeed be difficult, it is not at all impossible. Alternatives to foam fractionation exist, although these are often considered to be inefficient, unpredictable, time consuming and laborious. These include the Deep Sand Bed (DSB), in which heterotrophic bacteria remove nitrogen, the Algal Turf Scrubber (ATS), which allows for the export of nitrogen and phosphorus via controlled growth of algae, nitrate-reducing sulphur reactors, in which autotrophic bacteria remove nitrogen, and DyMiCo (Dynamic Mineral Control), a more advanced, computer-controlled DSB with active carbon injection. Some aquaria even run without any active filtration, and depend on regular water changes. These are often (very) small aquaria, making relatively large water changes feasible. Although a protein skimmer is not essential, it is a recommended filtration option for most hobbyists, who prefer high stocking densities and low maintenance.
Concluding remarks
Although the tone of this article may appear somewhat patronising, our hope is that it contributes to a better understanding of the marine life we maintain in our aquaria. We think that basic scientific knowledge and a slightly skeptical attitude will contribute to a successful aquarium hobby. In the end, we should all keep an open mind, but remain critical towards claims that have no factual basis.
About the authors

Tim has been working with corals since 2005, starting out in his student dormitory. After obtaining his Bachelor’s and Master’s degrees in biology at Utrecht University, he earned a Ph.D. in marine biology at Wageningen University, for which he studied coral physiology and feeding (image: N. Hurtado).

Arjen has been working with corals since 1999, and is now pursuing a Master’s degree in marine biology at the University of Groningen. He recently concluded a study on the effects of climate change on coral growth and physiology (image: A. Tilstra).
References
- Admiraal W (1984) The ecology of estuarine sediment inhabiting diatoms. ProgPhycol Res 3:269-322
- Allemand D, Ferrier-Pagès C, Furla P, Houlbrèque F, Puverel S, Reynaud S, Tambutté É, Tambutté S, Zoccola D (2004) Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. Comptes Rendus Palevol 3:453-467
- Baker AC (2001) Ecosystems: reef corals bleach to survive change. Nature411:765-766
- Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annual Rev Ecol Evol Syst34:661-689
- Baker AC, Starger CJ, McClanahan T, Glynn PW (2004) Corals’ adaptive response to climate change. Nature 430: 741
- Bare AY, Grimshaw KL, Rooney JJ,. Sabater MG, Fenner D, Carroll B (2010) Mesophotic communities of the insular shelf at Tutuila, American Samoa. Coral Reefs 29:369-377
- Bauman AG, Burt JA, Feary DA, Marquis E, Usseglio P (2010) Tropical harmful algal blooms: An emerging threat to coral reef communities? Mar Poll Bull 60:2117-2122
- Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc R Soc Lond B Biological Sciences 273:2305-2312
- Bongaerts P, Bridge TCL, Kline DI, Muir PR, Wallace CC, Beaman RJ, Hoegh-Guldberg O (2011) Mesophotic coral ecosystems on the walls of Coral Sea atolls. Coral Reefs 10.1007/s00338-011-0725-7
- Bou-Abdallah F, Chasteen ND, Lesser MP (2006) Quenching of superoxide radicals by green fluorescent protein. Biochim Biophys Acta 1760:1690-1695
- Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, Harvell CD (2009) Microbial disease and the coral holobiont. 17:554-562
- Bourne GC (1905) Report on the solitary corals collected by Professor Herdmann, at Ceylon, in 1902. Ceylon Pearl Oyster Fisheries, Part 4, Supplementary Report 29:187-242, pls. 1-4
- Brown BE, Dunne RP, Scoffin TP, Le Tissier MDA (1994) Solar damage in intertidal corals. Mar Ecol Prog Ser 105:30-43
- Budd AF, Fukami H, Smith ND, Knowlton N (2012) Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool J Linn Soc 166:465-529
- Carl M (2008) Predators and pests of captive corals, 31-36. In: Leewis RJ, Janse M (Eds) Advances in Coral Husbandry in Public Aquariums – Public Aquarium Husbandry Series, Volume 2, Burgers’ Zoo, Arnhem, The Netherlands. 444 p
- Chindapol N, Kaandorp JA, Cronemberger C, Mass T, Genin A (2013) Modelling Growth and Form of the Scleractinian Coral Pocillopora verrucosa and the Influence of Hydrodynamics. PLoS Comput Biol 9(1): e1002849. doi:10.1371/journal.pcbi.1002849
- Coffroth MA, Poland DM, Petrou EL, Brazeau DA, Holmberg JC (2010) Environmental Symbiont Acquisition May Not Be the Solution to Warming Seas for Reef-Building Corals. PLoS ONE 5(10):e13258
- Coffroth MA, Santos SR, Goulet TL (2001) Early ontogenic expression of specificity in a cnidarian-algal symbiosis. Mar Ecol Prog Ser222:85-96
- Cunning R, Baker AC (2012) Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat Clim Change, doi: 10.1038/nclimate1711
- D’Angelo C, Denzel A, Vogt A, Matz MV, Oswald F, Salih A, Nienhaus GU, Wiedenmann J (2008) Blue light regulation of host pigment in reef-building corals. Mar Ecol Prog Ser 364:97-106
- Dai C-F, Lin M-C (1993) The effects of flow on feeding of three gorgonians from southern Taiwan. J Exp Mar Biol Ecol 173:57-69
- Demmig-Adams B, Adams WW (1996) Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species. Planta 198:460-470
- Dubinsky Z, Stambler N, Ben-Zion M, McCloskey LR, Muscatine L, Falkowski PG (1990) The effect of external nutrient resources on the optical properties and photosynthetic efficiency of Stylophora pistillata. Proc R Soc Lond B Biological Sciences 239:231-246
- Dunlap WC, Shick JM (1998) Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef oganisms: a biochemical and environmental perspective. J Phycol 34:418-43
- Dunn JG, Sammarco PW, LaFleur G (2012) Effects of phosphate on growth and skeletal density in the scleractinian coral Acropora muricata: a controlled experimental approach. J Exp Mar Biol Ecol 411:34-44
- Ehrenberg CG (1834) Beiträge zur physiologischen Kenntniss der Corallenthiere im allgemeinen, und besonders des rothen Meeres, nebst einem Versuche zur physiologischen Systematik derselben. Abhandlungen der Königlichen Akademie der Wissenschaften, Berlin 1:225-380
- Enriquez S, Mendez ER, Iglesias-Prieto R (2005) Limnol Oceanogr 50(4):1025-1032
- Fabricius KE (2006) Effects of irradiance, flow and colony pigmentation on the temperature microenvironment around corals: implications for coral bleaching? Limnol Oceanogr 51:30-37
- Fabricius KE, Genin A, Benayahu Y (1995) Flow-dependent herbivory and growth in zooxanthellae-free soft corals. Limnol Oceanogr 40:1290-1301
- Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L (1993) Population control in symbiotic corals. Bioscience 43:606-611
- Ferrier-Pagès C, Gattuso JP, Dallot S, Jaubert J (2000) Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19:103-113
- Finelli CM, Helmuth BS, Pentcheff ND, Wethey DS (2007) Intracolony variability in photosynthesis by corals is affected by water flow: role of oxygen flux. Mar Ecol Prog Ser 349:103-110
- Finelli CM, Helmuth BST, Pentcheff ND, Wethey DS (2006) Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs 25:47-57
- Glynn PW, D’Croz L (1990) Experimental evidence for high temperature stress as the cause of El Niño-coincident coral mortality. Coral Reefs8:181-192
- Gorbunov MY, Falkowsky PG (2002) Photoreceptors in the Cnidarian Hosts Allow Symbiotic Corals to Sense Blue Moonlight. Limnol Oceanogr 47:309-315
- Goulet TL, Coffroth MA (2003) Stability of an octocoral-algal symbiosis over time and space. Mar Ecol Prog Ser 250:117-124
- Grottoli A, Rodrigues L, Palardy J (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186-1189
- Guzmán H, Cortés J, Glynn P, Richmond R (1990) Coral mortality associated with dinoflagellate blooms in the eastern Pacific (Costa Rica and Panama). Mar Ecol Prog Series. Oldendorf 60:299-303
- Hendelberg J, Åkesson B (1988) Convolutriloba retrogemma gen. et sp.n., a turbellarian (Acoela, Platyhelminthes) with reversed polarity of reproductive buds. Fortschr Zool 36:321-327
- Hill R, Frankart C, Ralph PJ (2005) Impact of bleaching conditions on the components of non-photochemical quenching in the zooxanthellae of a coral. J Exp Mar Biol Ecol 322:83-92
- Hoagland KD, Rosowski JR, Gretz MR, Roemer SC (1993) Diatom extracellular polymeric substances: function, fine structure, chemistry and physiology. J Phycol 29:537-556
- Hoeksema B (2013) Acroporidae Verrill, 1902. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=196095 on 2014-02-02
- Huang X-D, Dixon DG, Greenberg BM (1995) Increased polycyclic aromatic hydrocarbon toxicity following their photomodification in natural sunlight: Impacts on the duckweed Lemna gibba L. G3. Ecotoxicol Environ Saf 32:194-200
- Hylkema A, Wijgerde T, Osinga R (2014) in preparation
- Jimenez IM, Kühl M, Larkum ADW, Ralph PJ (2011) Effects of flow and colony morphology on the thermal boundary layer of corals. J R Soc Interface 8:1785-1795
- Jones AM, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair W (2008) A community shift in the symbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc R Soc B Biological Sciences275:1359-1365
- Jones RJ, Hoegh-Guldberg O (2001) Diurnal changes in the photochemical efficiency of symbiotic dinoflagellates (Dinophyceae) of corals: photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Environ 24:89-99
- Jury C (2006) Renaming our corals. Reefkeeping 5(3)
- Kinzie III RA (1993) Effects of ambient levels of solar ultraviolet radiation on zooxanthellae and photosynthesis of the reef coral Montipora verrucosa. Mar Biol 116:319-327
- Kinzie III RA, Jokiel PL, York R (1984) Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Mar Biol 78:239-248
- Kirda M (2003) Lighting In Reef Tanks: Some Actual Data. Advanced Aquarist 2(8).
- Le Tissier MDA, Brown BE (1996) Dynamics of solar bleaching in the intertidal reef coral Goniastrea aspera at Ko Phuket, Thailand. Mar Ecol Prog Ser 136:235-244
- Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Guldberg O (2007) Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Biol Ecol346:36-44
- Luna GM, Biavasco F, Danovaro R (2007) Bacteria associated with the rapid tissue necrosis of stony corals. Environm Microbiol 9:1851-1857
- Margulis L, Schwartz KV (1998) Five Kingdoms: an illustrated guide to the Phyla of life on earth. 3rd edition. Freeman: New York, USA. 520 p
- Marti-Puig, Forsman ZH, Haverkort-Yeh RD, Knapp ISS, Maragos JE, Toonen RJ (2014) Extreme phenotypic polymorphism in the coral genus Pocillopora; micro-morphology corresponds to mitochondrial groups, while colony morphology does not. Bull Mar Sci 90(1) dx.doi.org/10.5343/bms.2012.1080
- Martin KH, Knauer GA (1973) The elemental composition of plankton. Geochim Cosmochim Acta 37:1639-1653
- Marubini F, Davies PS (1996) Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar Biol 127:319-328
- Marubini F, Thake B (1999) Bicarbonate addition promotes coral growth. Limnol Oceanogr 44:716-720
- Mass T, Genin A, Shavit U, Grinstein M, Tchernov D (2010) Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc Nat Ac Sc USA 107:2527-2531
- Middelburg JJ, Barranguet C, Boschker HTS, Herman PMJ, Moens T, Heip CHR (2000) The fate of intertidal microphytobenthos carbon: an in situ 13C-labeling study. Limnol Oceanogr 45:1224-1234
- Mieog JC, van Oppen MJH, Cantin NE, Stam WT, Olsen JL (2007) Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs 26:449-457
- Mumby PJ, Steneck RS (2008) Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol 23:555-563
- Muscatine L, Falkowski PG, Dubinsky Z, Cook PA, McCloskey LR (1989) The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc R Soc Lond B Biological Sciences 236:311-324
- Nosratpour F (2008) Observations of a polyclad flatworm affecting acroporid corals in captivity. In: Leewis RJ, Janse M (Eds) Advances in Coral Husbandry in Public Aquariums – Public Aquarium Husbandry Series, Volume 2, Burgers’ Zoo, Arnhem, 37-46
- Osinga R, Schutter M, Griffioen B, Wijffels RH, Verreth JAJ, Shafir S, Henard S, Taruffi M, Gili C, Lavorano S (2011) The biology and economics of coral growth. Mar Biotechnol 13:658-671
- Pawlowsky J, Holzmann M, Fahrni JF, Pochon X, Lee JJ (2001) Molecular Identification of Algal Endosymbionts in Large Miliolid Foraminifera: 2. Dinoflagellates. J Eukary Microbiol 48:368-373
- Pochon X, Montoya-Burgos JI, Stadelmann B, Pawlowski J (2006) Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellate genus Symbiodinium. Mol Phyl Evol 38:20-30
- Purser A, Larsson AI, Thomsen L, van Oevelen D (2010) The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. J Exp Mar Biol Ecol 395:55-62
- Rawlinson KA, Gillis JA, Billings RE, Borneman EH (2011) Taxonomy and life history of the Acropora-eating flatworm Amakusaplana acroporae nov. sp. (Polycladida: Prosthiostomidae). Coral Reefs 30:693-705
- Reef R, Kaniewska P, Hoegh-Guldberg O (2009) Coral skeletons defend against ultraviolet radiation. PLoS ONE 4:e7995
- Richmond RH, Wolanski E (2011) Coral research: past efforts and future horizons. In: Dubinsky, Zvy, and Stambler, Noga, (eds.) Coral Reefs: An Ecosystem in Transition. Springer, London, pp. 3-10
- Riddle D (2007) How Much Light?! Analyses of Selected Shallow Water Invertebrates’ Light Requirements. Advanced Aquarist 6(3)
- Rooney J, Donham E, Montgomery A, Spalding H, Parrish F, Boland R, Fenner D, Gove J, Vetter O (2010) Mesophotic coral ecosystems in the Hawaiian Archipelago. Coral Reefs 10.1007/s00338-010-0596-3
- Rosenberg E, Ben-Haim Y (2002) Microbial diseases of corals and global warming. Environ Microbiol 4:318-326
- Rowan R (2004) Thermal adaptation in reef coral symbionts. Nature430:742
- Salih A, Larkum A, Cox G, Kuhl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408:850-853
- Schmidt-Roach S, Miller KJ, Lundgren P, Andreakis N (2014) With eyes wide open: A revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) based on morphology and genetics. Zool J Linn Soc 170:1-33
- Schutter M, Kranenbarg S, Wijffels RH, Verreth JAJ, Osinga R (2011) Modification of light utilization for skeletal growth by water flow in the scleractinian coral Galaxea fascicularis. Mar Biol 158:769-777
- Schutter M, Van Velthoven B, Janse M, Osinga R, Janssen M, Wijffels R, Verreth J (2008) J Exp Mar Biol Ecol 67:75-80
- Shannon T, Achatz JG (2007) Convolutriloba macropyga sp. nov., an uncommonly fecund acoel (Acoelomorpha) discovered in tropical aquaria. Zootaxa 1525:1-17
- Spotte S (1992) Captive seawater fishes: science and technology. J.Wiley & Sons Inc. 942 p
- Stambler N, Popper N, Dubinsky Z, Stimson J (1991) Effects of nutrient enrichment and water motion on the coral Pocillopora damicornis. Pacific Science 45:299-307
- Tanaka Y, Miyajima T, Koike I, Hayashibara T, Ogawa H (2007) Imbalanced coral growth between organic tissue and carbonate skeleton caused by nutrient enrichment. Limnol Oceanogr 52:1139-1146
- Tilstra A (2012) Symbiodinium detection in CaCO3 contaminated samples from the scleractinian coral Acropora millepora. MSc mini project, University of Groningen, The Netherlands
- Tilstra A, Wijgerde T, Eriksson BDHK, Falcão Salles J (2014) Genotype-specific bleaching response to elevated temperatures of different light adapted colonies of the scleractinian coral Stylophora pistillata. MSc Thesis, University of Groningen, Netherlands
- Titlyanov E, Bil’ K, Fomina I, Titlyanova T, Leletkin V, Eden N, Malkin A, Dubinsky Z (2000) Effects of dissolved ammonium addition and host with Artemia salina on photoacclimation of the hermatypic coral Stylophora pistillata. Mar Biol 137:463-472
- Titlyanov EA, Titlyanova TV, Yamazato K, van Woesik R (2001a) Photoacclimation of the hermatypic coral Stylophora pistillata while subjected to either starvation or food provisioning. J Exp Mar Biol Ecol 257: 163-181
- Titlyanov EA, Titlyanova TV, Yamazato K, van Woesik R (2001b) Photo-acclimation dynamics of the coral Stylophora pistillata to low and extremely low light. J Exp Mar Biol Ecol 263:211-225
- Tuttle RC, Loeblich III AR (1975) An optimal growth medium for the dinoflagellate Crypthecodinium cohnii. Phycologia 14:1-8
- Ulstrup KE, van Oppen MJ (2003) Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol Ecol12:3477-3484
- Venn AA, Loram JE, Douglas AE (2008) Photosynthetic symbioses in animals. J Exp Bot 59:1069-1080
- Veron JEN (1995) Corals in space and time. Cornell University Press, New York
- Veron JEN (2011) Coral Taxonomy and Evolution. In: Dubinsky, Zvy, and Stambler, Noga, (eds.) Coral Reefs: An Ecosystem in Transition. Springer, London, pp. 37-45
- Wagner D, Pochon X, Irwin L, Toonen RJ, Gates RD (2011) Azooxanthellate? Most Hawaiian black corals contain Symbiodinium. Proc R Soc Lond B Biological Sciences278:1323-1328
- Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Nat Ac Sc USA 96:8007-8012
- Wijgerde T (2012) Improved husbandry of marine invertebrates using an innovative filtration technology – part two: results with two 12 m3 DyMiCo systems. Advanced Aquarist 11(3)
- Wijgerde T (2013a) Coral feeding: an overview. Advanced Aquarist 12(12)
- Wijgerde T (2013b) Grow with the Flow. Reefs magazine 7(1)
- Wijgerde T, Laterveer M (2013) Coral growth under Light Emitting Diode and Light Emitting Plasma: a cross-family comparison. Advanced Aquarist 12(2)
- Wijgerde T, Schots P, van Onselen E, Janse M, Karruppannan E, Verreth JAJ, Osinga R (2012a) Epizoic acoelomorph flatworms impair zooplankton feeding by the scleractinian coral Galaxea fascicularis. Biol Open 2:10-17
- Wijgerde T, Spijkers P, Karruppannan E, Verreth JAJ, Osinga R (2012b) Water flow affects zooplankton feeding by the scleractinian coral Galaxea fascicularis on a polyp and colony level. J Mar Biol doi:10.1155/2012/854849
- Wijgerde T, Spijkers P, Verreth J, Osinga R (2011) Epizoic acoelomorph flatworms compete with their coral host for zooplankton. Coral Reefs 30:665
- WoRMS (2013) Acoela incertae sedis. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=380104 on 2014-01-15
- Yallop ML, De Winder B, Paterson DM, Stal LJ (1994) Comparative structure, primary production and biogenic stabilization of cohesive and non-cohesive marine sediments inhabited by microphytobenthos. Estuar Coast Shelf Sc 39:565-582



Great article. All who desire to keep a Reef Tank need to understand the topics covered here.